Inflammatory and Nutritional Biomarkers in Patients With Esophageal Squamous Cell Carcinoma Undergoing Neoadjuvant Chemotherapy and Radiation Therapy
Objectives: To investigate the relationship between pretreatment inflammatory and nutritional biomarkers in patients with esophageal squamous cell carcinoma (ESCC) undergoing neoadjuvant chemotherapy and radiation therapy (nCRT).
Sample & Setting: 213 patients with newly diagnosed stage II–III ESCC who received nCRT at an academic hospital in Taiwan.
Methods & Variables: Electronic health record data were used. Records on inflammatory and nutritional biomarkers and clinical outcomes were extracted. Logistic regression analysis was used to predict treatment-related adverse events, Cox regression was used for survival outcomes, and receiver operating characteristic curve analysis was used to determine optimal cutoff values.
Results: There was a significant association between low prognostic nutritional index (PNI) and nCRT toxicities and survival. Advanced cancer stage, high platelet-to-lymphocyte ratio, and occurrence of pneumonia/infection were linked to survival outcomes.
Implications for Nursing: PNI shows promise in predicting prognosis, helps identify high-risk patients, and enables nurses to apply tailored interventions.
Jump to a section
Based on World Health Organization (2022) estimates, esophageal cancer is the seventh most frequently diagnosed malignant neoplasm and the sixth leading cause of cancer-related mortality worldwide. In 2023, about 21,560 cases were anticipated to be diagnosed, with 16,120 expected fatalities from this disease (Siegel et al., 2023). The pathologic subtypes of esophageal cancer exhibit distinct geographic distribution patterns. Esophageal squamous cell carcinoma (ESCC) is predominantly observed in East Asia, such as in Taiwan, and is particularly prominent in the Asian Esophageal Cancer Belt region on the world map; its prevalence is expected to rise because of increased tobacco smoking, betel nut chewing, and alcohol consumption (Chen, Chen, et al., 2022). The significant geographic and histologic variations in esophageal cancer incidence rates pose challenges in comprehending its pathophysiology and management. Surgery continues to be the primary treatment for early-stage esophageal cancer. Patients with stage I cancer typically undergo surgery alone or in combination with chemotherapy and radiation therapy (CRT) (Lagergren et al., 2017). For locally advanced ESCC, neoadjuvant CRT (nCRT) followed by surgery is the standard approach (Ajani et al., 2019; Muro et al., 2019). However, despite recent advancements in interprofessional interventions, the quality of life of patients with esophageal cancer remains substantially decreased, and the overall prognosis remains unfavorable, with a five-year survival rate of 30% (Sudo et al., 2021).
For resectable advanced esophageal cancer (stages II and III), nCRT is the predominant form of treatment in Taiwan (Ho et al., 2018). About 60% of patients receiving CRT may encounter substantial side effects. Radiation therapy induces effects such as odynophagia, esophageal narrowing, and esophagitis (De Ruysscher et al., 2019). Chemotherapy also results in myelosuppression, anorexia, and vomiting (Wang, Dai, et al., 2023). These side effects can result in reduced CRT tolerance, prolonged hospital stays, and diminished quality of life, thereby potentially affecting overall survival (OS) (Wang, Li, et al., 2023; Wong & Law, 2017). Research indicates that sufficient nutritional support and effective management are crucial factors in enhancing immune function, reducing complications, and improving the overall prognosis of patients (Jordan et al., 2018). In addition, nurses play a critical role in guiding patients through nCRT. Identifying predictive factors for severe adverse events (AEs) and long-term prognosis is necessary for more tailored patient management to prevent unplanned acute care events among individuals undergoing cancer treatment (Osterman et al., 2022).
Esophageal cancer tumor progression and prognosis in patients are influenced by tumor pathology, pretreatment systemic inflammation, and nutritional status (Li et al., 2022; Liu et al., 2017; Liu & Lin, 2019; Watanabe et al., 2020). In the context of tumor biology, inflammation assumes a pivotal role in the tumor initiation, proliferation, and metastasis (Grivennikov et al., 2010; Han et al., 2020). The magnitude of inflammation can be indirectly explored via the measurement of systemic inflammation blood-based indicators, such as neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), and monocyte-to-lymphocyte ratio, that have emerged as valuable predictors of prognosis in esophageal cancer (Li et al., 2022; Liu et al., 2017; Liu & Lin, 2019). Malnutrition is more prevalent in esophageal cancer compared with other tumor types, particularly in patients with locally advanced disease (Okada et al., 2021). In addition, malnutrition can significantly influence the treatment tolerance and prognosis of patients with esophageal cancer (Cao et al., 2021). When oral intake is insufficient to meet the nutritional needs, alternative enteral routes are recommended (Wang, Dai, et al., 2023). Numerous studies have used prognostic nutritional index (PNI) as a prognostic indicator for esophageal cancer (Aoyama et al., 2023; Hao et al., 2020; Liao et al., 2020; Xue et al., 2019). PNI, derived from serum albumin levels and total circulating lymphocyte counts, reflects the nutritional and immunologic status of patients with cancer (Yan et al., 2022). In the context of PNI composition, albumin is crucial to facilitating nutrition transport and participating in metabolic processes (Infusino & Panteghini, 2013). In addition, lymphocytes encompassing B and T lymphocytes and their effector cells are essential components of the adaptive immune response (Zhao et al., 2017). Higher NLR and PLR and lower PNI are correlated with poor prognosis in patients with ESCC (Chen, Lee, et al., 2022; Hao et al., 2020; Liao et al., 2020; Xue et al., 2019).
The present research framework consolidates the perspectives of inflammation and nutrition within the domain of cancer biology. The concept of low PNI was first identified by Buzby et al. (1980) as a predictor for postoperative complications following abdominal and thoracic surgeries. Onodera et al. (1984) later refined and applied PNI in gastrointestinal cancer surgery for malnourished patients. Considering the significance of inflammation and nutritional status in oncology practice and application, the theoretical framework of the current work was inspired by Hsueh et al. (2022), incorporating the use of PNI and various inflammatory markers for predicting survival. In addition, the outcome variables in this work extend beyond survival status to include the prediction of treatment-related AEs and infection events.
To date, investigations on the link of pretreatment NLR, PLR, and PNI with the incidence of acute toxicity, complications, or infection events during nCRT in patients with ESCC are scarce. The relationship between treatment-related AEs and survival in patients with ESCC also needs to be more adequately established. To address the gaps in the literature, the present study primarily aimed to investigate the association of pretreatment NLR, PLR, and PNI with the nCRT-related toxicity and survival outcomes of patients with ESCC who underwent nCRT. The findings have implications for nursing care because they highlight the significance of assessing crucial predictive factors that promptly identify high-risk patients. In addition, the application of assessment results during the initial stages of nCRT can be instrumental in strengthening interprofessional collaboration aiming to reduce subsequent complications.
Methods
Study Design
The current study used a retrospective design, extracting data from the electronic health records of newly diagnosed patients with ESCC who underwent nCRT between January 1, 2013, and December 31, 2019. The study design followed the guidelines provided by the STROBE (Vandenbroucke et al., 2007) checklist for cohort studies (von Elm et al., 2008). This study obtained ethical approval from the institutional review board of the Research Ethics Committee at Hualien Tzu Chi Hospital and Buddhist Tzu Chi Medical Foundation (IRB110-082-B). Given the retrospective and observational nature of this research, the requirement for informed consent was waived.
Sample and Setting
This study was conducted in a 1,000-bed general hospital in an academic medical center at Hualien Tzu Chi Hospital in Taiwan. About 65 patients with ESCC are treated in the hospital annually. Eligible patients included in the study were diagnosed with ESCC stages II–III. Cancer staging was performed according to the seventh edition of the American Joint Committee on Cancer staging manual (Rice et al., 2010). All patients were treated with the same nCRT regimen. The nCRT regimen (Herskovic et al., 1992) included radiation therapy with a median prescribed dose of 45 Gy (range = 40–50.4 Gy) and a frequency of five fractions per week. Patients underwent two (29-day) cycles of chemotherapy. The authors excluded patients with a prior history of cancer, active infections, or immunodeficiency conditions (i.e., HIV or hepatitis B/C carriers), as well as those who were transferred or had incomplete medical records (i.e., missing treatment details or physiological parameters) or a gap of more than 14 days between chemotherapy and radiation therapy initiation. Esophagectomy was scheduled for four to eight weeks after completion of nCRT. Following the practices of this medical center, the nutritional care protocol suggested jejunostomy placement for patients with dietary energy intake below 20 kcal/kg/day for more than a week before nCRT. A dietitian provided weekly nutritional counseling to assess the nutritional status and support needs of patients with esophageal cancer during nCRT.
Procedures and Measures
Data for eligible patients were acquired from the Cancer Center of Hualien Tzu Chi Hospital. All variable data were obtained from the electronic health records of the patients. Baseline demographic and clinical characteristics were obtained from patients initially admitted for chemotherapy. This information included age at diagnosis, sex, smoking history, alcohol consumption, and betel nut chewing. Comorbidity information was further abstracted from the medical records and assessed using the Charlson Comorbidity Index following the transformation rule outlined in a prior study (Deyo et al., 1992). Data on the tumor location of ESCC (upper, middle, and lower segments), clinical tumor stage according to the seventh edition of the American Joint Committee on Cancer staging manual, and jejunostomy tube placement status (i.e., with or without) were also obtained. Pretreatment inflammatory and nutritional biomarkers were collected within one week before nCRT. NLR and PLR were derived from complete blood count and differential count, calculated as the ratios of neutrophil and platelet count to lymphocyte counts, respectively (Yodying et al., 2016). A higher score indicates severe inflammatory status. PNI was determined by adding the albumin value to five times the lymphocyte count (Nakatani et al., 2017). Low PNI may result from reduced albumin levels and/or decreased lymphocyte counts.
Outcome variables included the treatment-related AEs grade 3 or greater and survival status. The AEs were assessed at least weekly during nCRT and graded using the National Cancer Institute Cancer Therapy Evaluation Program (2017) Common Terminology Criteria for Adverse Events, version 5.0. Infection event occurrences were recorded starting two days after the initiation of nCRT and continued to be recorded for a period of six weeks following the completion of radiation therapy. Infection was established based on the identification of at least one positive culture for bacteria or fungi, and the inception of infection was defined as the date when positive culture findings were obtained. For patients who had multiple cultures positive for the same organism, infection events were considered independent if they occurred within 30 days. Regarding polymicrobial infections, each isolated causative organism was considered a separate infection event. The recording period for pneumonia occurrence is consistent with the collection of infection events. Pneumonia was defined by hospitalizations assigned a discharge diagnosis, an outpatient visit, or an emergency department visit with a pneumonia code range of 480–488 according to the International Classification of Diseases, Ninth Revision (Higgins et al., 2020). Survival status was determined for OS and disease-free survival (DFS). Patients were followed up until death or December 31, 2020, whichever occurred first. OS was defined as the time from nCRT until death from any cause, whereas DFS was defined as the time from nCRT to the date of first relapse, progression, or death from any cause.
Data Analysis
According to the formal guidance on sample size calculations for real-world retrospective medical chart review studies, a minimum sample size of 200 is required (Johnston et al., 2019). For variables with a skewed distribution, such as age, albumin, PNI, NLR, and PLR, median and interquartile range were used. Categorical variables were expressed as number and percentage. The association between baseline characteristics and nCRT-related severe AEs was assessed using logistic regression analysis. Cox regression analysis was performed for baseline characteristics, biomarkers, and AEs regarding mortality. When continuous variables, such as NLR, PLR, and PNI, exhibited significant predictive value in multivariate regression, receiver operating characteristic curve analysis was used for determining the optimal cutoff value based on Youden’s (1950) index. The Cox regression model of OS and DFS was used to explore the association between the baseline characteristics, severe AEs, and survival outcomes of patients. A p value of < 0.05 was considered statistically significant. Analyses were performed using IBM SPSS Statistics, version 28.0.
Results
Baseline Demographic and Clinical Characteristics
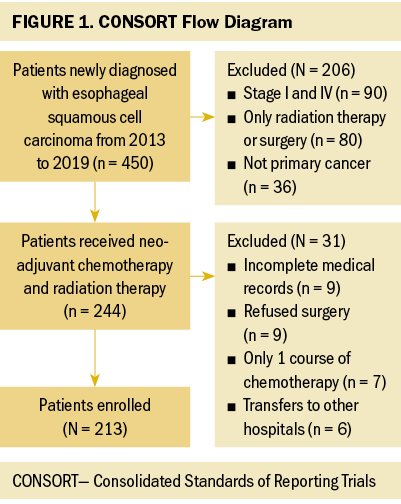
A total of 244 patients with ESCC stages II and III who underwent nCRT from January 1, 2013, to December 31, 2019, were identified for this study. Thirty-one patients were excluded because of incomplete medical records, refused surgery, only one course of chemotherapy, and transfers to other hospitals. Figure 1 illustrates the flow diagram of the included patients. Finally, 213 patients (192 men and 21 women) were evaluated. Table 1 displays the baseline characteristics of the patients.
Baseline Characteristics Associated With the Severity of AEs in Chemotherapy and Radiation Therapy
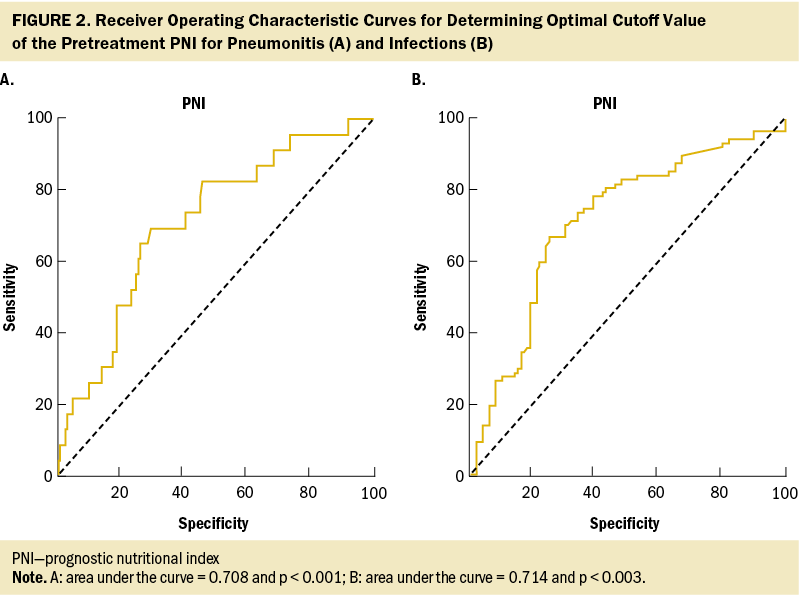
Table 2 lists the treatment toxicities of nCRT. The most common grade 3 hematologic toxicities were neutropenia, and two patients (1%) experienced grade 4 anemia. The most common grade 3 nonhematologic toxicity was mucositis. In addition, 82 patients (39%) experienced grade 3 infections, and 22 patients (10%) experienced grade 3 pneumonia. The primary source of wound infection was predominantly attributed to the jejunostomy site (data not shown). Based on these findings, severe AEs with an incidence rate exceeding 10% and associated with significant clinical mortality risk factors, such as neutropenia, pneumonia, and infections, were included in the regression model. The construction of receiver operating characteristic curves for PNI in relation to pneumonia and infection events resulted in optimal cutoff values of 38 and 40, respectively, with an area under the curve of 0.7 (see Figure 2). Table 3 presents the findings of the univariate and multivariate logistic analyses. The multivariate logistic regression showed that age (odds ratio [OR] = 3.03, 95% confidence interval [CI] [1.05, 8.7], p = 0.039) and PNI (OR = 2.25, 95% CI [1.09, 4.98], p = 0.008) are associated with pneumonia. PNI (OR = 3, 95% CI [1.6, 5.59], p = 0.001) and jejunostomy placement (OR = 3.19, 95% CI [1.6, 6.68], p = 0.001) are predictors for infections.
Survival Analysis
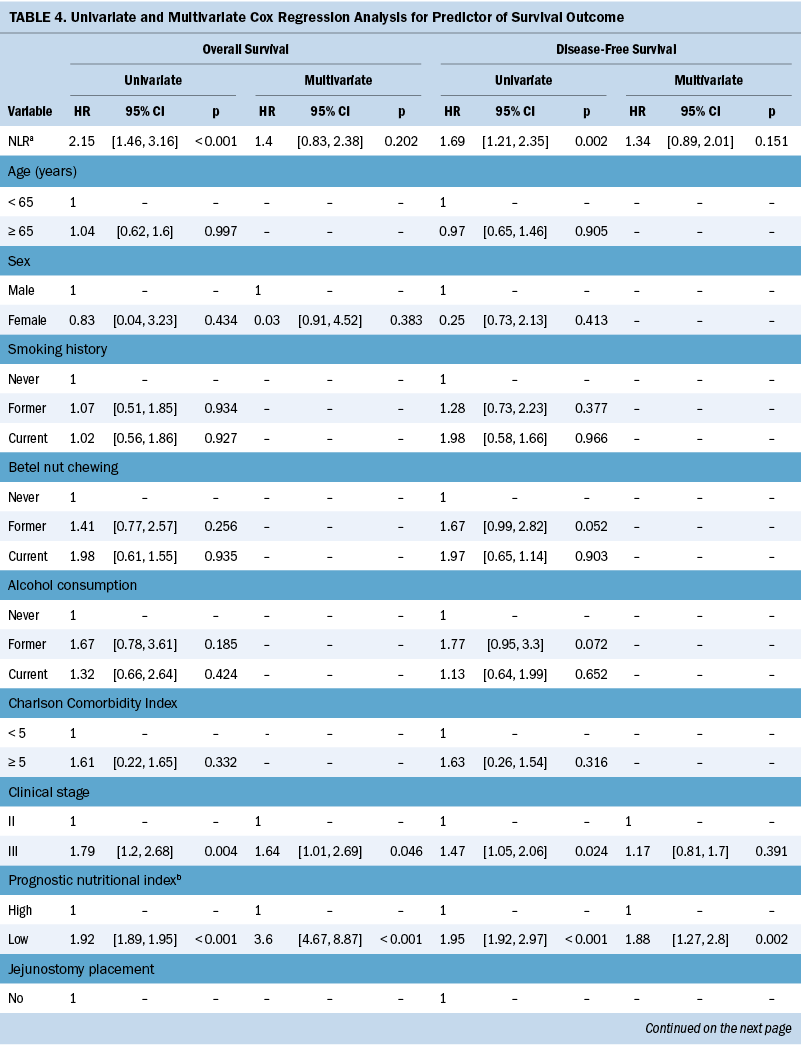
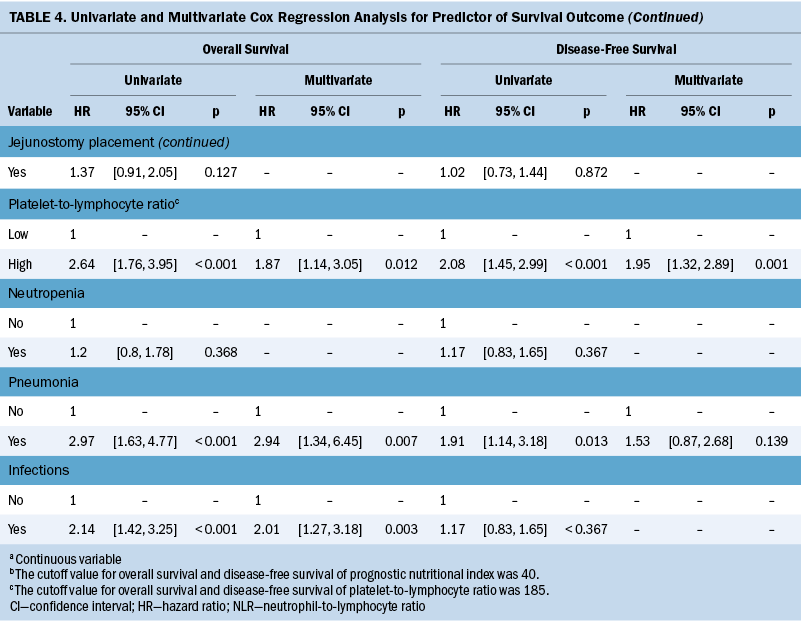
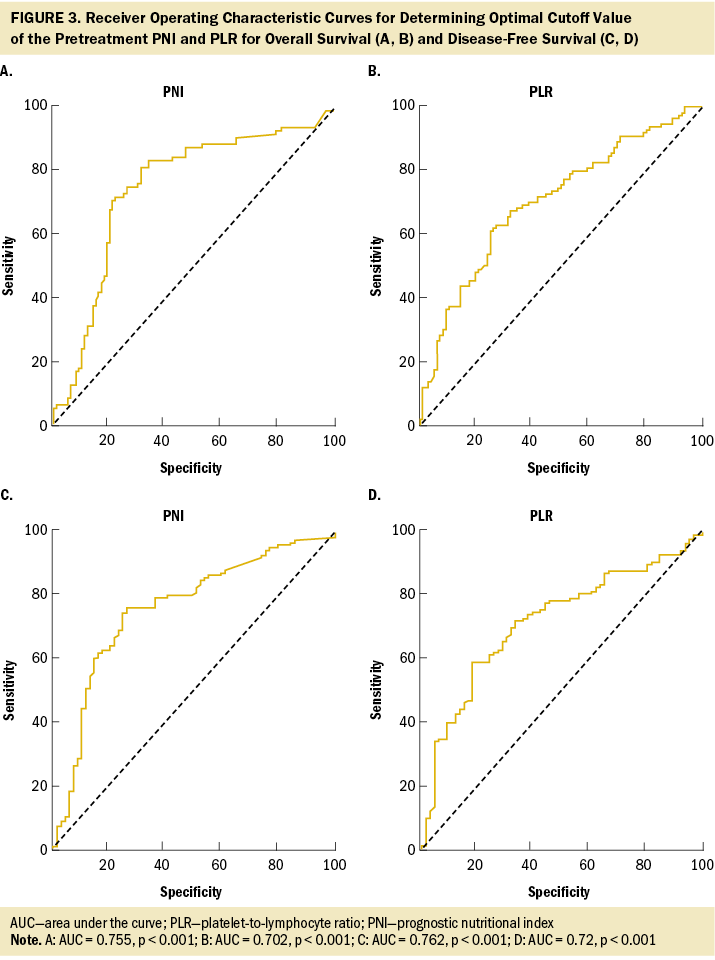
The median OS and DFS periods were 30.7 months (range = 6.8–88.4 months) and 20.4 months (range = 6.1–71.9 months), respectively. Significant baseline characteristics and severe AEs in the univariate analysis were fitted into the multivariate Cox regression model, as shown in Table 4. The cutoff values for OS and DFS were 40 and 185, respectively, for patients with low PNI and those with a high PLR (see Figure 3). Multivariate Cox regression model showed that clinical stage III (hazard ratio [HR] = 1.64, 95% CI [1.01, 2.69], p = 0.046), low PNI (HR = 3.6, 95% CI [4.67, 8.87], p < 0.001), high PLR (HR = 1.87, 95% CI [1.14, 3.05], p = 0.012), pneumonia (HR = 2.94, 95% CI [1.34, 6.45], p = 0.007), and infections (HR = 2.01, 95% CI [1.27, 3.18], p = 0.003) are predictors of OS. Pretreatment low PNI (HR = 1.88, 95% CI [1.27, 2.8], p = 0.002) and high PLR (HR = 1.95, 95% CI [1.32, 2.89], p = 0.001) are predictors of DFS.
Discussion
In the retrospective cohort study of 213 patients with ESCC undergoing nCRT, the association between inflammatory and nutritional biomarkers, treatment-related AEs, and survival outcomes was investigated. Low PNI is a predictor of nCRT-related toxicities and poor survival, highlighting its robust correlation with unfavorable prognosis in patients with ESCC. Regarding OS, advanced cancer stage, low PNI, high PLR, and pneumonia or infection during nCRT are significant factors. Pretreatment PNI and PLR are prognostic factors for predicting DFS. Integrating PNI and PLR assessment before treatment, along with monitoring AEs during esophageal cancer care, enables proactive complication prediction and real-time management. Previous studies (Basch et al., 2017; Denis et al., 2019) suggest that the nursing responses to monitoring AEs during treatment are associated with improved survival. Early responsiveness to patient symptoms and AEs may help avert adverse outcomes, enhancing treatment tolerance and survival.
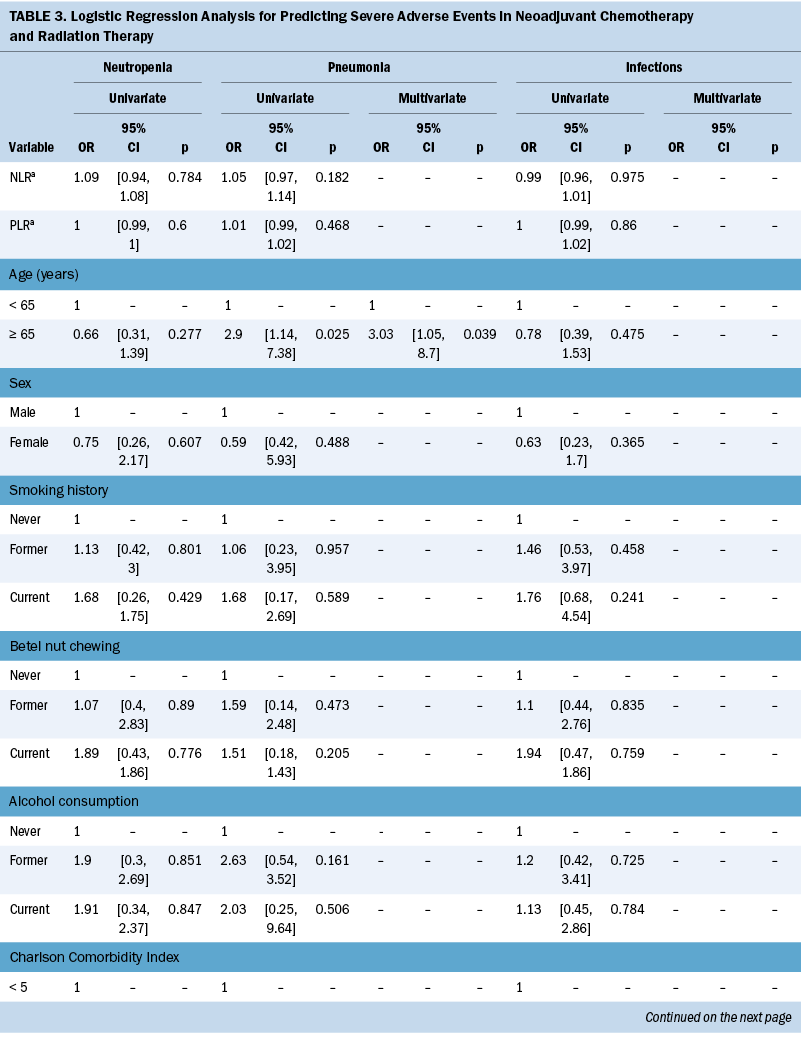
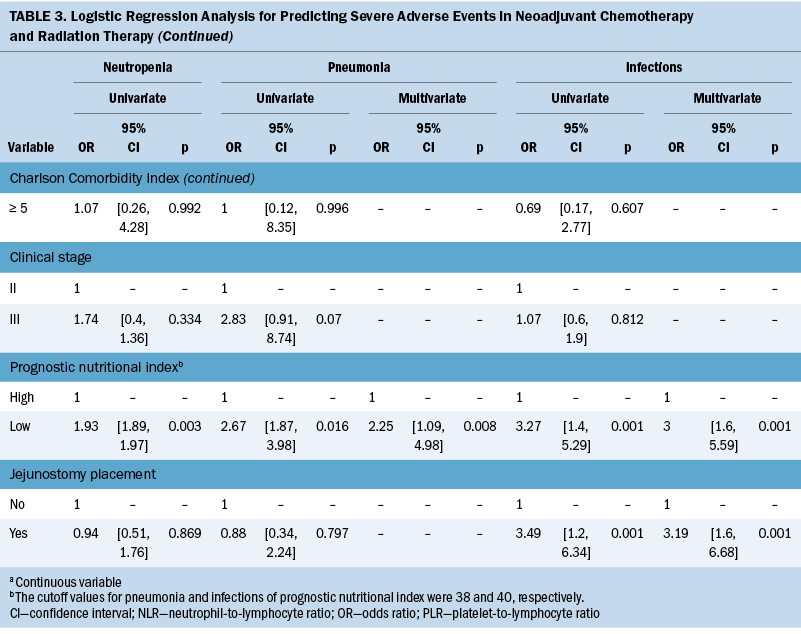
The nCRT-related AE incidence in the current study was in line with a previous Cochrane review (Kidane et al., 2015), where reported rates of grade 3 or higher treatment-related toxicities ranged from 11% to more than 38%. Neutropenia emerges as the most common AE in patients undergoing chemotherapy with myelosuppressive drugs (Tralongo et al., 2020). However, no relation was found between neutropenia and risk factors in this study. A possible reason is that the authors’ center follows the guidelines and administers granulocyte– colony-stimulating factor for neutropenia prophylaxis (Klastersky et al., 2016; Tralongo et al., 2020). The supportive use of granulocyte–colony-stimulating factor reduces the frequency and severity of neutropenia in patients undergoing treatment with myelosuppressive drugs (Becker et al., 2020).
In this study, low PNI is associated with the development of pneumonia complications and infections. However, to the authors’ knowledge, the underlying mechanism explaining the correlation between PNI, AEs during nCRT, and survival status in patients with ESCC remains unclear. PNI is determined by evaluating serum albumin concentration and total lymphocyte count in peripheral blood. Although the exact mechanisms of lower PNI could not be determined in the current study, several hypotheses could help explain these findings. The significant prognostic value of PNI in ESCC outcomes can be attributed to several mechanisms. First, hypoalbuminemia may result from malnutrition because of the heightened metabolic burden resulting from adverse reactions and inadequate nutrient intake and leading to poorer physical condition and unfavorable treatment-related AEs (Movahed et al., 2021). Second, lymphocytes play crucial roles in combating cancer by initiating cytotoxic immune responses and inhibiting cancer cell growth, invasion, and spread (Grivennikov et al., 2010). Lymphocytopenia is linked to unfavorable clinical outcomes in patients with diverse cancer types (Ray-Coquard et al., 2009). Finally, poor nutrition and weakened immunity can enhance the spread of circulating tumor cells (Sakurai et al., 2016), whereas PNI reflects tumor progression (Feng & Chen, 2014).
Malnutrition is closely associated with inflammation, compromised immunity, and heightened infection susceptibility (Merker et al., 2020). In addition, tumors induce structural changes in the esophagus, increasing the likelihood of irritation and severe coughing post–ingestion of substances. Subsequent esophageal radiation therapy poses inherent risks to the trachea and bronchus, potentially softening these structures with higher radiation doses and elevating the risk of pneumonia (Abugroun et al., 2017). Collectively, these factors contribute to the increased occurrence of infections or pneumonia during treatment. Findings suggest PNI has potential utility in predicting outcomes among patients with infections (Hu et al., 2021). Most prior studies focused on predicting postoperative pneumonia in patients with esophageal cancer (Fujishima et al., 2021; Fukushima et al., 2023). The present study is the first to discover, aside from age, an association between low PNI and the occurrence of pneumonia during nCRT. In addition to low PNI, the occurrence of pneumonia and infection events is associated with unfavorable survival outcomes. AEs associated with CRT can cause delays or discontinuation of treatment because of complications, leading to prolonged surgical duration, heightened surgical complexities, and diminished quality of life in patients with ESCC, potentially resulting in unfavorable outcomes (De Ruysscher et al., 2019). In the present study, PNI can predict the occurrence of nCRT-related AEs and the survival outcomes of patients with ESCC. This predictive ability stems from the PNI’s capacity to quantify the immune nutritional status of individual patients throughout the treatment course.
Previous studies suggested that inflammatory biomarkers are significantly associated with complications and AEs for patients with cancer receiving multimodal treatment (Li et al., 2022; Liu et al., 2017; Liu & Lin, 2019), but the related studies for patients with ESCC treated with nCRT are very limited. A previous study (Cai et al., 2020) reported that pretreatment NLR is an independent factor for predicting grade 3 or greater hematologic toxicity in patients with ESCC undergoing nCRT. This study is inconsistent with previous findings, possibly because of their approach of summing various types of hematologic toxic events into a single predictive event, the discrepancies in treatment regimens, and histologic types. In this study, PLR failed to predict AEs, but the predictive value for survival outcomes was retained. This finding is consistent with the results of several previous meta-analysis studies (Ishibashi et al., 2021; Zhang et al., 2018). The procoagulant surface presented by platelet cells in cancer-related coagulation can encapsulate tumor cells, shielding against anticancer immunity and ultimately facilitating tumor growth (Bambace & Holmes, 2011). PLR may be associated with tumor microenvironmental inflammation, which, in turn, is linked to tumor progression, thereby influencing prognosis (Ohno et al., 2019).
Clinical evidence supports the benefits of rational nutritional therapy in increasing nutritional reserves, maintaining physical fitness and tolerance, reducing complications, and accelerating recovery from esophageal cancer (Lyu et al., 2022). According to the findings of the current study, the placement of a jejunostomy tube may not be associated with survival rate and may increase the risk of infections. Enteral nutrition is the preferred method of nutrition for patients with esophageal cancer who have some level of gastrointestinal function but encounter challenges with oral food intake, and various factors may contribute to an increased risk of infection in these patients. Despite the recognized clinical advantages of preventive tube feeding, a significant portion of patients decline its usage because of the discomfort associated with the process of intubation (Wang, Dai, et al., 2023). Feeding jejunostomy–related complications include dislodgement, leakage, jejunostomy site inflammation, and jejunostomy obstruction (Kim et al., 2022). Previous research demonstrated that dysphagia can show improvement from baseline to the completion of the first cycle of chemotherapy (Chilukuri et al., 2018). In addition, advancements in esophagectomy procedures have markedly reduced the necessity of jejunostomy (Berkelmans et al., 2018). Studies also indicated that the use of feeding jejunostomy delays—rather than prevents—weight loss, thereby undermining the potential nutritional benefits of intraoperative feeding jejunostomy (Carroll et al., 2020; Tham et al., 2020).
Variations in PNI during nCRT have a significant effect on survival and treatment response. Takao et al. (2020) demonstrated that patients with initially low PNI values who experienced an increase in PNI at the end of treatment have significantly higher survival rates compared with patients who maintained low PNI values at the end of treatment. Previous research indicated the beneficial effects of omega-3 polyunsaturated fatty acids in immunonutrition formulations in attenuating inflammation in patients with esophageal cancer (Eltweri et al., 2017; Kanekiyo et al., 2019). In a meta-synthesis (Wang, Liu, et al., 2023), it was found that nutritional intervention during treatment and recovery posed challenges. Healthcare providers should address symptoms related to digestive discomfort, eating conditions, and emotional well-being. Further clinical trials are needed to develop comprehensive nutritional management systems, establish interprofessional teams, create favorable nutritional environments, and optimize nutritional therapy for patients with esophageal cancer.
Limitations
First, this work is a retrospective observational study conducted solely at an academic medical center, which may introduce bias from the retrospective review and limit the generalizability of the findings. To mitigate selection bias, the current authors received thorough training and adhered to the criteria established in prior studies when selecting research participants (Hsueh et al., 2022). Of note, the present study’s sample size is larger than that in Hsueh et al. (2022). Second, the collection of PNI, NLR, and PLR at a single time point during nCRT may hinder the authors’ understanding of their dynamic changes during treatment and their potential effect on prognosis. Despite the lack of a universally accepted standard for the optimal cutoff values of PNI and PLR, in this study, accurate thresholds were determined using the receiver operating characteristic curve, which is consistent with prior research in predicting infections, pneumonia, and survival outcomes (Hsueh et al., 2022; Suzuki et al., 2020). In addition, the lack of detailed information on nutritional supplementation may affect the predictive power of treatment-related AEs and survival outcomes.
Implications for Nursing
PNI holds significant implications for nursing practice, serving as a valuable and noninvasive tool. Its simplicity, speed, and user-friendliness enable its use as a laboratory data indicator for enhancing nurses’ clinical alertness judgment. The following strategies are proposed to implement standardized nursing care incorporating PNI in a clinical setting. First, a calculated PNI field is integrated into electronic health records and nursing care documentation. Second, engagement in interprofessional discussions is encouraged to collectively interpret PNI results and thus facilitate the identification of appropriate interventions for ensuring a smoother recovery during nCRT and minimizing adverse reactions. For patients using a jejunostomy tube, remaining vigilant about associated complications is also essential. Nursing staff can customize care plans, potentially improving patients’ clinical outcomes through observation of high-risk patients and effective teamwork.

Conclusion
The current study investigated pretreatment inflammatory and nutritional biomarkers in nCRT-related AEs and long-term outcomes of patients with ESCC. The results showed significant associations between low PNI and pneumonia and infections. In addition, pretreatment with low PNI has been linked to poorer survival outcomes and may indicate a greater risk of recurrence in ESCC. This study is believed to be the first to investigate the association of PNI with infections and pneumonia in patients undergoing nCRT. Additional research could involve nutritional intervention to determine whether the benefits of improved nutritional status can decrease poor prognosis outcomes.
About the Authors
Chun Hou Huang, PhD, ANP, is an assistant professor in the Department of Nursing at Tzu Chi University, Bee Song Chang, MD, is the chief of thoracic surgery in the Department of Thoracic Surgery at Hualien Buddhist Tzu Chi General Hospital, and Tai-Chu Peng, PhD, RN, is a professor in the Department of Nursing at Tzu Chi University, all in Hualien, Taiwan; and Yun-Hsin Peng, MSN, RN, is a clinical nurse in the Department of Nursing at National Taiwan University Hospital in Taipei. This research was funded, in part, by the Tzu Chi Medical Mission Project 109-04, Buddhist Tzu Chi Medical Foundation (TCMMP 109-04). Huang, Chang, and T.-C. Peng contributed to the conceptualization and design, provided statistical support and the analysis, and contributed to the manuscript preparation. All authors completed the data collection. Huang can be reached at hou2017@gms.tcu .edu.tw, with copy to ONFEditor@ons.org. (Submitted August 2023. Accepted December 19, 2023.)
References
Abugroun, A., Ahmed, F., Singh, N., & Nadiri, M. (2017). Late onset chemo/radiation induced tracheoesophageal fistula in squamous cell cancer of the lung. World Journal of Oncology, 8(5), 171–173. https://doi.org/10.14740/wjon1063w
Ajani, J.A., D’Amico, T.A., Bentrem, D.J., Chao, J., Corvera, C., Das, P., . . . Pluchino, L.A. (2019). Esophageal and esophagogastric junction cancers, version 2.2019, NCCN Clinical Practice Guidelines in Oncology. Journal of the National Comprehensive Cancer Network, 17(7), 855–883. https://doi.org/10.6004/jnccn.2019.0033
Aoyama, T., Kazama, K., Maezawa, Y., & Hara, K. (2023). Usefulness of nutrition and inflammation assessment tools in esophageal cancer treatment. In Vivo, 37(1), 22–35. https://doi.org/10.21873/invivo.13051
Bambace, N.M., & Holmes, C.E. (2011). The platelet contribution to cancer progression. Journal of Thrombosis and Haemostasis, 9(2), 237–249. https://doi.org/10.1111/j.1538-7836.2010.04131.x
Basch, E., Deal, A.M., Dueck, A.C., Scher, H.I., Kris, M.G., Hudis, C., & Schrag, D. (2017). Overall survival results of a trial assessing patient-reported outcomes for symptom monitoring during routine cancer treatment. JAMA, 318(2), 197–198. https://doi.org/10.1001/jama.2017.7156
Becker, P.S., Griffiths, E.A., Alwan, L.M., Bachiashvili, K., Brown, A., Cool, R., . . . Pluchino, L.A. (2020). NCCN Guidelines insights: Hematopoietic growth factors, version 1.2020. Journal of the National Comprehensive Cancer Network, 18(1), 12–22. https://doi.org/10.6004/jnccn.2020.0002
Berkelmans, G.H.K., Fransen, L., Weijs, T.J., Lubbers, M., Nieuwenhuijzen, G.A.P., Ruurda, J.P., . . . Luyer, M.D.P. (2018). The long-term effects of early oral feeding following minimal invasive esophagectomy. Diseases of the Esophagus, 31(1), 1–8. https://doi.org/10.1093/dote/dox114
Buzby, G.P., Mullen, J.L., Matthews, D.C., Hobbs, C.L., & Rosato, E.F. (1980). Prognostic nutritional index in gastrointestinal surgery. American Journal of Surgery, 139(1), 160–167. https://doi.org/10.1016/0002-9610(80)90246-9
Cai, G., Yu, J., & Meng, X. (2020). Predicting prognosis and adverse events by hematologic markers in patients with locally advanced esophageal squamous cell carcinoma treated with neoadjuvant chemoradiotherapy. Cancer Management and Research, 12, 8497–8507. https://doi.org/10.2147/cmar.S257058
Cao, J., Xu, H., Li, W., Guo, Z., Lin, Y., Shi, Y., . . . Shi, H. (2021). Nutritional assessment and risk factors associated to malnutrition in patients with esophageal cancer. Current Problems in Cancer, 45(1), 100638. https://doi.org/10.1016/j.currproblcancer.2020.100638
Carroll, P.A., Yeung, J.C., & Darling, G.E. (2020). Elimination of routine feeding jejunostomy after esophagectomy. Annals of Thoracic Surgery, 110(5), 1706–1713. https://doi.org/10.1016/j.athoracsur.2020.04.072
Chen, C.-J., Lee, C.-T., Tsai, Y.-N., Tseng, C.-M., Chen, T.-H., Hsu, M.-H., . . . Wang, W.-L. (2022). Prognostic significance of systemic inflammatory response markers in patients with superficial esophageal squamous cell carcinomas. Scientific Reports, 12(1), 18241. https://doi.org/10.1038/s41598-022-21974-y
Chen, H.Y., Chen, I.C., Chen, Y.H., Chen, C.C., Chuang, C.Y., & Lin, C.H. (2022). The influence of socioeconomic status on esophageal cancer in Taiwan: A population-based study. Journal of Personalized Medicine, 12(4), 595. https://doi.org/10.3390/jpm12040595
Chilukuri, P., Odufalu, F., & Hachem, C. (2018). Dysphagia. Missouri Medicine, 115(3), 206–210.
Denis, F., Basch, E., Septans, A.-L., Bennouna, J., Urban, T., Dueck, A.C., & Letellier, C. (2019). Two-year survival comparing web-based symptom monitoring vs routine surveillance following treatment for lung cancer. JAMA, 321(3), 306–307. https://doi.org/10.1001/jama.2018.18085
De Ruysscher, D., Niedermann, G., Burnet, N.G., Siva, S., Lee, A.W.M., & Hegi-Johnson, F. (2019). Radiotherapy toxicity. Nature Reviews. Disease Primers, 5(1), 13. https://doi.org/10.1038/s41572-019-0064-5
Deyo, R.A., Cherkin, D.C., & Ciol, M.A. (1992). Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. Journal of Clinical Epidemiology, 45(6), 613–619. https://doi.org/10.1016/0895-4356(92)90133-8
Eltweri, A.M., Thomas, A.L., Metcalfe, M., Calder, P.C., Dennison, A.R., & Bowrey, D.J. (2017). Potential applications of fish oils rich in omega-3 polyunsaturated fatty acids in the management of gastrointestinal cancer. Clinical Nutrition, 36(1), 65–78. https://doi.org/10.1016/j.clnu.2016.01.007
Feng, J.-F., & Chen, Q.-X. (2014). Significance of the prognostic nutritional index in patients with esophageal squamous cell carcinoma. Therapeutics and Clinical Risk Management, 10, 1–7. https://doi.org/10.2147/tcrm.S56159
Fujishima, S., Tsujimoto, H., Nagata, K., Sugasawa, H., Nomura, S., Ito, N., . . . Ueno, H. (2021). Postoperative pneumonia causes the loss of skeletal muscle volume and poor prognosis in patients undergoing esophagectomy for esophageal cancer. General Thoracic and Cardiovascular Surgery, 69(1), 84–90. https://doi.org/10.1007/s11748-020-01482-4
Fukushima, T., Watanabe, N., Okita, Y., Yokota, S., Matsuoka, A., Kojima, K., . . . Daiko, H. (2023). The evaluation of the association between preoperative sarcopenia and postoperative pneumonia and factors for preoperative sarcopenia in patients undergoing thoracoscopic-laparoscopic esophagectomy for esophageal cancer. Surgery Today, 53(7), 782–790. https://doi.org/10.1007/s00595-022-02620-6
Grivennikov, S.I., Greten, F.R., & Karin, M. (2010). Immunity, inflammation, and cancer. Cell, 140(6), 883–899. https://doi.org/10.1016/j.cell.2010.01.025
Han, W., Allam, S.A., & Elsawa, S.F. (2020). GLI2-mediated inflammation in the tumor microenvironment. Advances in Experimental Medicine and Biology, 1263, 55–65. https://doi.org/10.1007/978-3-030-44518-8_5
Hao, J., Chen, C., Wan, F., Zhu, Y., Jin, H., Zhou, J., . . . Pu, Q. (2020). Prognostic value of pre-treatment prognostic nutritional index in esophageal cancer: A systematic review and meta-analysis. Frontiers in Oncology, 10, 797. https://doi.org/10.3389/fonc.2020.00797
Herskovic, A., Martz, K., al-Sarraf, M., Leichman, L., Brindle, J., Vaitkevicius, V., . . . Emami, B. (1992). Combined chemotherapy and radiotherapy compared with radiotherapy alone in patients with cancer of the esophagus. New England Journal of Medicine, 326(24), 1593–1598.
Higgins, T.L., Deshpande, A., Zilberberg, M.D., Lindenauer, P.K., Imrey, P.B., Yu, P.C., . . . Rothberg, M.B. (2020). Assessment of the accuracy of using ICD-9 diagnosis codes to identify pneumonia etiology in patients hospitalized with pneumonia. JAMA Network Open, 3(7), e207750. https://doi.org/10.1001/jamanetworkopen.2020.7750
Ho, H.-J., Chen, H.-S., Hung, W.-H., Hsu, P.-K., Wu, S.-C., Chen, H.-C., & Wang, B.-Y. (2018). Survival impact of total resected lymph nodes in esophageal cancer patients with and without neoadjuvant chemoradiation. Annals of Surgical Oncology, 25(13), 3820–3832. https://doi.org/10.1245/s10434-018-6785-y
Hsueh, W.H., Hsueh, S.-W., Yeh, K.-Y., Hung, Y.-S., Ho, M.-M., Lin, S.-Y., . . . Chou, W.-C. (2022). Albumin and neutrophil-to-lymphocyte ratio score in neoadjuvant concurrent chemoradiotherapy for esophageal cancer: Comparison with prognostic nutritional index. In Vivo, 36(5), 2400–2408. https://doi.org/10.21873/invivo.12973
Hu, X., Deng, H., Wang, Y., Chen, L., Gu, X., & Wang, X. (2021). Predictive value of the prognostic nutritional index for the severity of coronavirus disease 2019. Nutrition, 84, 111123. https://doi.org/10.1016/j.nut.2020.111123
Infusino, I., & Panteghini, M. (2013). Serum albumin: Accuracy and clinical use. International Journal of Clinical Chemistry, 419, 15–18. https://doi.org/10.1016/j.cca.2013.01.005
Ishibashi, Y., Tsujimoto, H., Sugasawa, H., Kouzu, K., Itazaki, Y., Sugihara, T., . . . Ueno, H. (2021). Prognostic value of platelet-related measures for overall survival in esophageal squamous cell carcinoma: A systematic review and meta-analysis. Critical Reviews in Oncology/Hematology, 164, 103427. https://doi.org/10.1016/j.critrevonc.2021.103427
Johnston, K.M., Lakzadeh, P., Donato, B.M.K., & Szabo, S.M. (2019). Methods of sample size calculation in descriptive retrospective burden of illness studies. BMC Medical Research Methodology, 19(1), 9. https://doi.org/10.1186/s12874-018-0657-9
Jordan, T., Mastnak, D.M., Palamar, N., & Kozjek, N.R. (2018). Nutritional therapy for patients with esophageal cancer. Nutrition and Cancer, 70(1), 23–29. https://doi.org/10.1080/01635581.2017.1374417
Kanekiyo, S., Takeda, S., Iida, M., Nishiyama, M., Kitahara, M., Shindo, Y., . . . Nagano, H. (2019). Efficacy of perioperative immunonutrition in esophageal cancer patients undergoing esophagectomy. Nutrition, 59, 96–102. https://doi.org/10.1016/j.nut.2018.08.006
Kidane, B., Coughlin, S., Vogt, K., & Malthaner, R. (2015). Preoperative chemotherapy for resectable thoracic esophageal cancer. Cochrane Database of Systematic Reviews, 2015(5), CD001556. https://doi.org/10.1002/14651858.CD001556.pub3
Kim, M.S., Shin, S., Kim, H.K., Choi, Y.S., Zo, J.I., Shim, Y.M., & Cho, J.H. (2022). Role of intraoperative feeding jejunostomy in esophageal cancer surgery. Journal of Cardiothoracic Surgery, 17(1), 191. https://doi.org/10.1186/s13019-022-01944-1
Klastersky, J., de Naurois, J., Rolston, K., Rapoport, B., Maschmeyer, G., Aapro, M., & Herrstedt, J. (2016). Management of febrile neutropaenia: ESMO clinical practice guidelines. Annals of Oncology, 27(Suppl. 5), v111–v118.
Lagergren, J., Smyth, E., Cunningham, D., & Lagergren, P. (2017). Oesophageal cancer. Lancet, 390(10110), 2383–2396. https://doi.org/10.1016/s0140-6736(17)31462-9
Li, X., Zhang, S., Lu, J., Li, C., & Li, N. (2022). The prognostic value of systemic immune-inflammation index in surgical esophageal cancer patients: An updated meta-analysis. Frontiers in Surgery, 9, 922595. https://doi.org/10.3389/fsurg.2022.922595
Liao, G., Zhao, Z., Yang, H., Chen, M., & Li, X. (2020). Can prognostic nutritional index be a prediction factor in esophageal cancer?: A meta-analysis. Nutrition and Cancer, 72(2), 187–193. https://doi.org/10.1080/01635581.2019.1631859
Liu, X., Li, M., Zhao, F., Zhu, Y., Luo, Y., Kong, L., . . . Yu, J. (2017). The lymphocyte-monocyte ratio predicts tumor response and survival in patients with locally advanced esophageal cancer who received definitive chemoradiotherapy. OncoTargets and Therapy, 10, 871–877. https://doi.org/10.2147/ott.S124915
Liu, Y.-H., & Lin, Y.-S. (2019). Platelet-lymphocyte and neutrophil-lymphocyte ratios: Predictive factors of response and toxicity for docetaxel-combined induction chemotherapy in advanced head and neck cancers. Journal of the Chinese Medical Association, 82(11), 849–855. https://doi.org/10.1097/jcma.0000000000000178
Lyu, J., Shi, A., Li, T., Li, J., Zhao, R., Zhu, S., . . . Shi, H. (2022). Effects of enteral nutrition on patients with oesophageal carcinoma treated with concurrent chemoradiotherapy: A prospective, multicentre, randomised, controlled study. Frontiers in Oncology, 12, 839516. https://doi.org/10.3389/fonc.2022.839516
Merker, M., Felder, M., Gueissaz, L., Bolliger, R., Tribolet, P., Kägi-Braun, N., . . . Schuetz, P. (2020). Association of baseline inflammation with effectiveness of nutritional support among patients with disease-related malnutrition: A secondary analysis of a randomized clinical trial. JAMA Network Open, 3(3), e200663. https://doi.org/10.1001/jamanetworkopen.2020.0663
Movahed, S., Varshoee Tabrizi, F., Pahlavani, N., Seilanian Toussi, M., Motlagh, A., Eslami, S., . . . Norouzy, A. (2021). Comprehensive assessment of nutritional status and nutritional-related complications in newly diagnosed esophageal cancer patients: A cross-sectional study. Clinical Nutrition, 40(6), 4449–4455. https://doi.org/10.1016/j.clnu.2021.01.003
Muro, K., Lordick, F., Tsushima, T., Pentheroudakis, G., Baba, E., Lu, Z., . . . Douillard, J.-Y. (2019). Pan-Asian adapted ESMO clinical practice guidelines for the management of patients with metastatic oesophageal cancer: A JSMO-ESMO initiative endorsed by CSCO, KSMO, MOS, SSO and TOS. Annals of Oncology, 30(1), 34–43. https://doi.org/10.1093/annonc/mdy498
Nakatani, M., Migita, K., Matsumoto, S., Wakatsuki, K., Ito, M., Nakade, H., . . . Kanehiro, H. (2017). Prognostic significance of the prognostic nutritional index in esophageal cancer patients undergoing neoadjuvant chemotherapy. Diseases of the Esophagus, 30(8), 1–7. https://doi.org/10.1093/dote/dox020
National Cancer Institute Cancer Therapy Evaluation Program. (2017). Common Terminology Criteria for Adverse Events [v.5.0]. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/doc…
Ohno, F., Nakahara, T., Kido-Nakahara, M., Ito, T., Nunomura, S., Izuhara, K., & Furue, M. (2019). Periostin links skin inflammation to melanoma progression in humans and mice. International Journal of Molecular Sciences, 20(1), 169. https://doi.org/10.3390/ijms20010169
Okada, G., Matsumoto, Y., Habu, D., Matsuda, Y., Lee, S., & Osugi, H. (2021). Relationship between GLIM criteria and disease-specific symptoms and its impact on 5-year survival of esophageal cancer patients. Clinical Nutrition, 40(9), 5072–5078. https://doi.org/10.1016/j.clnu.2021.08.008
Onodera, T., Goseki, N., & Kosaki, G. (1984). [Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients]. Nihon Geka Gakkai Zasshi, 85(9), 1001–1005.
Osterman, C.K., Sanoff, H.K., Wood, W.A., Fasold, M., & Lafata, J.E. (2022). Predictive modeling for adverse events and risk stratification programs for people receiving cancer treatment. JCO Oncology Practice, 18(2), 127–136. https://doi.org/10.1200/op.21.00198
Ray-Coquard, I., Cropet, C., Van Glabbeke, M., Sebban, C., Le Cesne, A., Judson, I., . . . Blay, J.-Y. (2009). Lymphopenia as a prognostic factor for overall survival in advanced carcinomas, sarcomas, and lymphomas. Cancer Research, 69(13), 5383–5391. https://doi.org/10.1158/0008-5472.Can-08-3845
Rice, T.W., Blackstone, E.H., & Rusch, V.W. (2010). 7th edition of the AJCC cancer staging manual: Esophagus and esophagogastric junction. Annals of Surgical Oncology, 17(7), 1721–1724. https://doi.org/10.1245/s10434-010-1024-1
Sakurai, K., Ohira, M., Tamura, T., Toyokawa, T., Amano, R., Kubo, N., . . . Hirakawa, K. (2016). Predictive potential of preoperative nutritional status in long-term outcome projections for patients with gastric cancer. Annals of Surgical Oncology, 23(2), 525–533. https://doi.org/10.1245/s10434-015-4814-7
Siegel, R.L., Miller, K.D., Wagle, N.S., & Jemal, A. (2023). Cancer statistics, 2023. CA: A Cancer Journal for Clinicians, 73(1), 17–48. https://doi.org/10.3322/caac.21763
Sudo, N., Ichikawa, H., Muneoka, Y., Hanyu, T., Kano, Y., Ishikawa, T., . . . Wakai, T. (2021). Clinical utility of ypTNM stage grouping in the 8th edition of the American Joint Committee on Cancer TNM staging system for esophageal squamous cell carcinoma. Annals of Surgical Oncology, 28(2), 650–660. https://doi.org/10.1245/s10434-020-09181-3
Suzuki, T., Ishibashi, Y., Tsujimoto, H., Nomura, S., Kouzu, K., Itazaki, Y., . . . Ueno, H. (2020). A novel systemic inflammatory score combined with immunoinflammatory markers accurately reflects prognosis in patients with esophageal cancer. In Vivo, 34(6), 3705–3711. https://doi.org/10.21873/invivo.12218
Takao, K., Konishi, H., Fujiwara, H., Shiozaki, A., Shoda, K., Kosuga, T., . . . Otsuji, E. (2020). Clinical significance of prognostic nutritional index in the treatment of esophageal squamous cell carcinoma. In Vivo, 34(6), 3451–3457. https://doi.org/10.21873/invivo.12184
Tham, J.C., Dovell, G., Berrisford, R.G., Humphreys, M.L., Wheatley, T.J., Sanders, G., & Ariyarathenam, A.V. (2020). Routine use of feeding jejunostomy in oesophageal cancer resections: Results of a survey in England. Diseases of the Esophagus, 33(4), doz075. https://doi.org/10.1093/dote/doz075
Tralongo, A.C., Antonuzzo, A., Pronzato, P., Sbrana, A., Turrini, M., Zoratto, F., & Danova, M. (2020). Management of chemotherapy-induced neutropenia in patients with cancer: 2019 guidelines of the Italian Medical Oncology Association (AIOM). Tumori, 106(4), 273–280. https://doi.org/10.1177/0300891620927093
Vandenbroucke, J.P., von Elm, E., Altman, D.G., Gøtzsche, P.C., Mulrow, C.D., Pocock, S.J., . . . Egger, M. (2007). Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): Explanation and elaboration. Annals of Internal Medicine, 147(8), W163–W194.
von Elm, E., Altman, D.G., Egger, M., Pocock, S.J., Gøtzsche, P.C., & Vandenbroucke, J.P. (2008). [The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting of observational studies]. Internist, 49(6), 688–693. https://doi.org/10.1007/s00108-008-2138-4
Wang, S.-A., Dai, W.-S., Zhu, J.-Y., Gao, B., Ren, W., & Chen, X. (2023). Nasogastric tube feeding improves nutritional status and physical state in esophageal cancer patients during chemoradiotherapy: A retrospective study. Supportive Care in Cancer, 31(6), 341. https://doi.org/10.1007/s00520-023-07780-w
Wang, S.-A., Li, F., Zhu, J., Chen, X., Ren, W., & Gao, B. (2023). Multidisciplinary nutritional management improves nutritional and hospitalized outcomes of patients with esophageal cancer undergoing chemoradiotherapy: A randomized control trial. Medicine, 102(12), e33335. https://doi.org/10.1097/md.0000000000033335
Wang, X., Liu, X., Gu, Z., Li, X., & Shu, Y. (2023). Experiences and requirements in nutritional management of patients with esophageal cancer: A systematic review and qualitative meta-synthesis. Supportive Care in Cancer, 31(12), 633. https://doi.org/10.1007/s00520-023-08100-y
Watanabe, M., Otake, R., Kozuki, R., Toihata, T., Takahashi, K., Okamura, A., & Imamura, Y. (2020). Recent progress in multidisciplinary treatment for patients with esophageal cancer. Surgery Today, 50(1), 12–20. https://doi.org/10.1007/s00595-019-01878-7
Wong, C., & Law, S. (2017). Predictive factors in the evaluation of treatment response to neoadjuvant chemoradiotherapy in patients with advanced esophageal squamous cell cancer. Journal of Thoracic Disease, 9(Suppl. 8), S773–S780. https://doi.org/10.21037/jtd.2017.04.29
World Health Organization. (2022). Oesophagus. http://gco.iarc.fr/today/data/factsheets/cancers/6-Oesophagus-fact-shee…
Xue, Y., Zhou, X., Xue, L., Zhou, R., & Luo, J. (2019). The role of pretreatment prognostic nutritional index in esophageal cancer: A meta-analysis. Journal of Cellular Physiology, 234(11), 19655–19662. https://doi.org/10.1002/jcp.28565
Yan, K., Wei, W., Shen, W., Du, X., Zhu, S., Zhao, H., . . . Deng, W. (2022). Combining the systemic inflammation response index and prognostic nutritional index to predict the prognosis of locally advanced elderly esophageal squamous cell carcinoma patients undergoing definitive radiotherapy. Journal of Gastrointestinal Oncology, 13(1), 13–25. https://doi.org/10.21037/jgo-21-784
Yodying, H., Matsuda, A., Miyashita, M., Matsumoto, S., Sakurazawa, N., Yamada, M., & Uchida, E. (2016). Prognostic significance of neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio in oncologic outcomes of esophageal cancer: A systematic review and meta-analysis. Annals of Surgical Oncology, 23(2), 646–654. https://doi.org/10.1245/s10434-015-4869-5
Youden, W.J. (1950). Index for rating diagnostic tests. Cancer, 3(1), 32–35. https://doi.org/10.1002/1097-0142(1950)3:1%3C32::aid-cncr2820030106%3E3…
Zhang, X., Wang, Y., Zhao, L., Sang, S., & Zhang, L. (2018). Prognostic value of platelet-to-lymphocyte ratio in oncologic outcomes of esophageal cancer: A systematic review and meta-analysis. International Journal of Biological Markers, 1724600818766889. https://doi.org/10.1177/1724600818766889
Zhao, W., Wang, P., Jia, H., Chen, M., Gu, X., Liu, M., . . . Wu, Z. (2017). Lymphocyte count or percentage: Which can better predict the prognosis of advanced cancer patients following palliative care? BMC Cancer, 17(1), 514. https://doi.org/10.1186/s12885-017-3498-8


