The Multifactorial Model of Cancer-Related Cognitive Impairment
Problem Identification: Cancer-related cognitive impairment (CRCI) is common and is associated with cancer and its treatments. Evidence suggests that the causes are multifactorial, but the field is lacking a comprehensive conceptual model of CRCI to summarize existing knowledge and provide a way to understand and predict causal links, as well as to generate hypotheses.
Literature Search: PubMed® and Google Scholar™ were searched, and 130 articles demonstrated several lacking factors needed for a more comprehensive CRCI model.
Data Evaluation: The new multifactorial model of CRCI includes social determinants of health, patient-specific factors, co-occurring symptoms, treatment factors, and biologic mechanisms.
Synthesis: The multifactorial model of CRCI is based on established and emerging evidence. This model is inclusive of all cancer types and associated treatments.
Implications for Nursing: Although it would be ideal to evaluate all the concepts and components in this model in a comprehensive fashion, investigators with existing datasets could evaluate portions of the model to determine directionality for some of the proposed relationships. The new model can be used to design preclinical and clinical studies of CRCI. Knowledge of the occurrence of CRCI and factors that contribute to this symptom will allow nurses to perform assessments of modifiable and nonmodifiable risk factors.
Jump to a section
Cognitive changes associated with cancer and its treatments, known as cancer-related cognitive impairment (CRCI), are reported by about 45% of survivors and patients receiving treatments for cancer (Schmidt et al., 2016; Wefel et al., 2014). Although chemotherapy is one factor (Ren et al., 2019), evidence suggests that the causes and mechanisms of various cognitive changes are multifactorial (Bai & Yu, 2021; Mampay et al., 2021; Yang & Hendrix, 2018). Because several cognitive domains are affected (Ren et al., 2019), CRCI results in decrements in activities of daily living (Boykoff et al., 2009), personal- (Potrata et al., 2010) and work-related responsibilities (Lange, Licaj, et al., 2019), and financial (Boykoff et al., 2009), emotional, and social well-being (Rust & Davis, 2013).
Despite efforts by the International Cognition and Cancer Task Force to harmonize assessment methods (Wefel et al., 2011), conceptual and empirical issues in CRCI research remain (Horowitz et al., 2018). Conceptually, neuropsychological tests may not detect the subtle changes and specific cognitive processes associated with CRCI (Horowitz et al., 2018). Empirical issues include the following absences: a universal definition of CRCI, a standard battery of subjective and objective measures to diagnose CRCI and monitor changes throughout time, and a correlation between neuropsychological test results and subjective reports of CRCI (Horowitz et al., 2018).
An equally important issue is the absence of a comprehensive conceptual model of CRCI. A conceptual model provides a visualization of the relationships among a set of concepts (i.e., variables that can be empirically observed or measured) that are thought to be linked to a phenomenon (Earp & Ennett, 1991). As a result, a conceptual model summarizes existing knowledge and provides a way to understand or predict causal links and to generate hypotheses (Earp & Ennett, 1991).
Hess and Insel (2007) published a conceptual model of CRCI that was specific to chemotherapy. It proposed that changes in cognitive function may occur along two different but interacting pathways (i.e., psychosocial effects of a cancer diagnosis and direct physiologic effects of the cancer treatment). Antecedents (e.g., cancer treatment) and consequences (e.g., decreased quality of life) of CRCI were identified, as well as various mediators and moderators. Although informative, this model is limited because it focused on cognitive changes only in patients who received chemotherapy.
This model was updated by Myers (2009) to include integration of the Theory of Unpleasant Symptoms. Because patients with cancer experience multiple co-occurring symptoms (Miaskowski et al., 2014), the blending of the initial conceptual model with this middle-range theory allowed for an evaluation of CRCI within the context of potential effects from other symptoms. However, since these two models were published, research focused on CRCI has expanded exponentially.
Ahles and Hurria (2018) published a conceptual model that focused on predictors of CRCI in cancer survivors. This model highlighted the need to consider stress as a potential risk factor for CRCI. However, the model’s exclusive focus on survivors limits its application to patients actively receiving treatments or those with advanced stages of cancer.
Although previous models may be useful for select groups of patients, a more comprehensive conceptual model of CRCI is needed to guide future research throughout the continuum of care. Therefore, the purpose of this article is to present the multifactorial model of CRCI (MMCRCI), a conceptual model based on established and emerging evidence.
Development of the MMCRCI
Literature Review
The first step in the development of the MMCRCI was a comprehensive review of the literature that identified factors (i.e., risk, protective, and mechanistic) associated with CRCI. The search was inclusive of all types of cancer and associated treatments. Pediatric studies were excluded because the factors associated with CRCI may differ in this age group. In addition, cognitive changes associated with oncologic emergencies (e.g., hypercalcemia of malignancy) were excluded because effective management of an oncologic emergency generally resolves associated cognitive changes (Klemencic & Perkins, 2019).
The following keywords and phrases were searched using PubMed® and Google Scholar™: cancer-related cognitive impairment; chemotherapy-related cognitive impairment; cancer-associated cognitive dysfunction; cancer AND cognition OR cognitive. In PubMed, search terms were mapped to their respective MeSH (Medical Subject Headings) for expanded results when possible. Keyword searches were supplemented by hand searches in the reference lists of relevant articles. More than 130 state-of-the-science or systematic review articles published between 2017 and 2021 were identified. These reviews were the primary sources of evidence for the development of the MMCRCI. In addition, some of the emerging evidence in the model is supported by studies of other types of cognitive impairment that warrant evaluation in patients with cancer.
Conceptual Organization of the MMCRCI
Once the factors associated with CRCI were identified, they were organized into broader concepts. As illustrated in Figure 1, the specific concepts in the MMCRCI are social determinants of health (SDOH), patient-specific factors, co-occurring symptoms, treatment factors, and biologic mechanisms. The directionality of many of these associations between the concepts and CRCI is not well established, although the various concepts interact throughout the continuum of care. The time points when assessments of CRCI are done are likely to affect the relationships between the various concepts in the MMCRCI because these concepts and their inter-relationships are dynamic in nature.
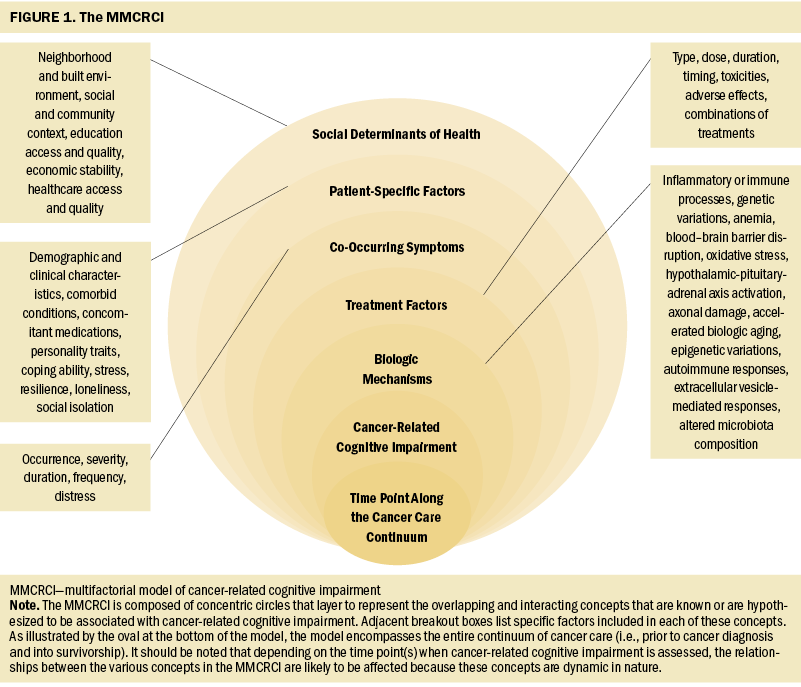
Assumptions of the MMCRCI
The underlying assumptions of the MMCRCI are as follows: (a) The causes and consequences of cognitive changes associated with cancer and its treatments are multifactorial; (b) these cognitive changes need to be evaluated in the context of multiple contributing factors; and (c) knowledge of the underlying mechanisms of CRCI, as well as effective interventions to prevent and treat this symptom, will be identified based on research that uses this model. Although it is well documented that CRCI has a negative effect on a variety of patient outcomes (Hess & Insel, 2007), because these outcomes are distant from the underlying concepts that contribute to this symptom, they are not included in this model.
Operational Definition of CRCI
An operational definition is an essential component of any conceptual model because it serves to represent a concept as a variable that can be measured empirically (Chinn & Kramer, 2011). There are challenges defining CRCI because multiple cognitive domains are affected and a large amount of interindividual variability exists (Dijkshoorn et al., 2021; Janelsins et al., 2014). Often included in the definition of CRCI are the cognitive domains that are most affected (e.g., attention, concentration, memory, processing speed, executive function) (Mayo et al., 2021; Ren et al., 2019). Alternatively, symptoms associated with various cognitive changes are described (e.g., slow processing speed, inability to concentrate) (El-Agamy et al., 2019). Based on a synthesis of definitions from several articles (Ahles & Hurria, 2018; Hess & Insel, 2007; Janelsins et al., 2014; Kanaskie, 2012; Myers, 2009), the definition of CRCI for the MMCRCI is as follows: a temporary or persistent subjective or objective change in higher-order mental processes that occurs with cancer or its treatments.
Model Components
SDOH
According to the Office of Disease Prevention and Health Promotion (n.d.), SDOH are “the conditions in the environments where people are born, live, learn, work, play, worship, and age that affect a wide range of health, functioning, and quality-of-life outcomes and risks” (para. 1). Studies evaluating the role of SDOH in the occurrence or severity of CRCI are limited. As noted in two systematic reviews (Coughlin, 2019, 2020), multiple SDOH (e.g., neighborhood disadvantage, lower socioeconomic status, access to health care, lower levels of education) contribute to undesired outcomes associated with cancer. Therefore, SDOH inclusion in the MMCRCI is warranted. In addition, associations are documented between multiple SDOH (e.g., food insecurity, neighborhood economic disadvantage) and an increased risk of cognitive decline (Majoka & Schimming, 2021).
As noted by Ahles and Root (2018), the effects of cultural differences in cognitive styles or socioeconomic status on CRCI are examples of valuable information that is missing from CRCI research to date. Future studies need to include more diverse samples of patients to allow for an increased understanding of the relationships between SDOH and CRCI. One innovative approach to evaluate SDOH is the development and use of a polysocial risk score (Figueroa et al., 2020). This score would allow for the aggregation of multiple SDOH and an evaluation of their effects on CRCI.
Patient-Specific Factors
The next concept in the MMCRCI is patient-specific factors. These factors can affect the occurrence or persistence of CRCI throughout the cancer care continuum. Although age is the most common demographic characteristic evaluated, results are inconsistent in terms of its association with CRCI (Kim et al., 2020). Given that the majority of CRCI research has focused on women with breast cancer (Bernstein et al., 2017; García-Sánchez et al., 2020; Kim et al., 2020; Sousa et al., 2020; Yang & Hendrix, 2018), the occurrence and effects of CRCI in other genders warrants evaluation. Other demographic characteristics that are potential risk factors for CRCI include a decreased cognitive reserve and lower level of education (Lange, Joly, et al., 2019). Additional research is needed to assess for associations between pre-existing or developing comorbid conditions and CRCI. In terms of concomitant medications, although the use of medications for pain and depression is associated with CRCI (Hess & Insel, 2007), other types of medications (e.g., anxiolytics) warrant evaluation.
Decrements in physical activity may be an important risk factor for CRCI. Exercise increases the expression of neurotrophic and neuroprotective factors that have anti-inflammatory effects and contribute to hippocampal neurogenesis (Zimmer et al., 2016). Of note, exercise as an intervention for CRCI is an area of intense investigation (Campbell et al., 2020; Farahani et al., 2022; Myers et al., 2018; Schaffrath et al., 2017; Shahid & Kim, 2020). However, additional information is needed on the underlying mechanisms of this association, as well as the type, dose, and timing of exercise interventions.
Only one study evaluated for associations between personality traits and CRCI. In this study of patients with breast cancer (Hermelink et al., 2010), negative affectivity was associated with an increase in self-reported problems with cognition and attention. Although research focusing on personality traits in oncology is limited, in one review of associations between personality traits and cognitive abilities in older adults (Curtis et al., 2015), higher levels of openness were associated with better general cognitive ability, fluid ability, episodic memory, and verbal ability. These findings support the inclusion of personality traits in the MMCRCI.
Only one study evaluated the relationship between coping and CRCI in patients with cancer (Reid-Arndt & Cox, 2012). Findings suggest that avoidant coping styles mediate the relationships between stress and a worsened performance on neuropsychological tests in the domains of memory and verbal fluency. Although research in oncology is limited, previous research in patients with Parkinson disease found that a decrease in task-oriented coping was associated with cognitive impairment and that those with reduced task-oriented coping were at increased risk for depression, anxiety, and decrements in quality of life (Hurt et al., 2012). Additional investigations are needed on the effects of different coping styles on CRCI.
In terms of acute stress, a review of the potential role for self-regulation in the development of CRCI highlights preclinical research that suggests a bidirectional relationship between self-regulation and executive function (Arndt et al., 2013). The authors hypothesized that coping with cancer and its treatments creates demands on self-regulatory capacities. Energy spent on cancer-related stress and coping consumes and diverts mental energy from other cognitive functions and, subsequently, contributes to CRCI.
Andreotti et al. (2015) described potential associations between chronic stress and CRCI, and hypothesized that individuals with a history of chronic stress may have an increased allostatic load that results in physiologic changes in the prefrontal brain. These brain changes may lead to hypothalamic-pituitary-adrenal axis disruption that impairs one’s ability to adaptively cope with stress. In turn, the psychobiologic effects of cancer and associated treatments are amplified, placing patients at increased risk for CRCI.
Although definitions vary, psychological resilience generally refers to an exposure to adversity and a subsequent positive adaptation (Fletcher & Sarkar, 2013). However, sociocultural factors may influence how resilience is defined in different populations (Fletcher & Sarkar, 2013). Resilience may influence the risk for CRCI because of its association with other contributing factors (e.g., coping style, personality, stress perception) (Southwick et al., 2005), which warrant consideration in future studies.
Although research specific to CRCI is limited, in a study of the general population (Lara et al., 2019), loneliness and social isolation were associated with decrements in objectively measured cognitive function. In another study of older adults (Evans et al., 2018), social isolation was associated with poorer cognitive function, moderated by cognitive reserve. Equally important, COVID-19 mitigation efforts increased social isolation, leaving those with cognitive impairments at increased risk of a higher symptom burden (Alonso-Lana et al., 2020). These findings support the evaluation of loneliness and social isolation in the MMCRCI.
Co-Occurring Symptoms
The next concept in the MMCRCI is co-occurring symptoms. Critical components of this concept include the occurrence, severity, duration, frequency, and distress of each symptom. Co-occurring symptoms may preexist, develop because of the cancer and its treatments, or occur because of comorbid conditions. A large amount of interindividual variability exists in symptom experiences. Equally important, symptoms are dynamic within and across each of the concepts included in the MMCRCI. In a systematic review of longitudinal studies that evaluated self-reported CRCI (Kim et al., 2020), the most frequent moderators of CRCI were depressive symptoms and fatigue. Another review aimed to synthesize the research on a number of psychological symptoms associated with CRCI in patients with breast cancer (Yang & Hendrix, 2018). Depression was the symptom most frequently associated with CRCI, followed by anxiety, anxiety and depression, worry, undefined psychological distress, and mental fatigue. Psychological distress stimulates the hypothalamic-pituitary-adrenal axis and sympathetic nervous system (Cui et al., 2021), which triggers increased production of neuroendocrine substances (e.g., cortisol, dopamine) that may contribute to CRCI. Ongoing research is needed to understand how other co-occurring symptoms may affect CRCI (Janelsins et al., 2014).
Treatment Factors
The components in the treatment factors concept include the type of treatment as well as its dose, duration, timing (e.g., chronotherapy), associated toxicities or adverse effects, or combinations of other treatments. Several reviews have highlighted the potential mechanisms that may contribute to CRCI based on types of treatment (Chia et al., 2021; Du et al., 2021; Eide & Feng, 2020; García-Sánchez et al., 2020; Harrison et al., 2021; Joly et al., 2019; Mayo et al., 2021; Morgans et al., 2021; Turnquist et al., 2020).
Given that many types of cancer require combinations of treatments (Dijkshoorn et al., 2021; Hwang et al., 2021), the potential additive or synergistic effects of multiple sequential or concurrent treatments warrant consideration in a model of CRCI. In a meta-analysis of patients with colorectal cancer (Hwang et al., 2021), individuals who received a higher number of treatment modalities were more likely to self-report CRCI. Additional research is needed to understand the mechanisms by which cancer therapies affect cognition (Monje et al., 2020).
Biologic Mechanisms
The final concept in the model is biologic mechanisms. Given that the etiology of CRCI is multifactorial (Janelsins et al., 2014), numerous mechanisms may contribute to its occurrence, severity, and persistence.
A number of inflammatory mechanisms (e.g., signaling molecules carried by extracellular vesicles) are implicated as potential causes of pretreatment CRCI because the cancer induces the activation or production of cytokines (Mampay et al., 2021; Olson & Marks, 2019). These inflammatory responses may affect the central nervous system and contribute to neuroinflammation (Olson & Marks, 2019). One review noted that, throughout the cancer care continuum, the most frequently measured biomarkers of CRCI were inflammatory substances in plasma (Castel et al., 2017). Overall, findings from these studies suggest that the administration of chemotherapy dysregulates cytokine levels and has a negative effect on brain function that results in CRCI. In addition, higher levels of circulating proinflammatory cytokines may cross the blood–brain barrier and result in neurotoxic damage and associated behavioral symptoms (e.g., depression, fatigue) (Henneghan, 2016).
Genetic variations associated with increased susceptibility for CRCI include genetic loci involved in a variety of biologic processes (e.g., inflammation, DNA damage and repair) as well as genes associated with neuronal degeneration, repair, and transmission (Buskbjerg et al., 2019). As noted in one systematic review (Buskbjerg et al., 2019), although some evidence suggests that the apolipoprotein E4 allele is associated with increased risk of CRCI, other studies found no association. Studies evaluating other candidate genes are limited and yielded inconclusive results (Buskbjerg et al., 2019).
Anemia was one of the earliest mechanisms that was evaluated for its associations with CRCI (Hess & Insel, 2007). In some studies, increases in hemoglobin levels were associated with improvements in cognitive function (Jacobsen et al., 2004; Vearncombe et al., 2009); however, no associations were found in other studies (Hedayati et al., 2012; Vardy et al., 2014).
In terms of structural brain changes, findings from one systematic review of longitudinal neuroimaging studies in patients with breast cancer suggest that distinct patterns associated with structural, perfusion, and functional changes may begin shortly after the initiation of chemotherapy and persist beyond treatment (Sousa et al., 2020). These data suggest specific vulnerability in the frontal lobes. The authors of this review suggested that neuroimaging techniques may be more sensitive than neuropsychological tests to detect CRCI. Another systematic review summarized the findings from cross-sectional and longitudinal studies that examined structural neuroimaging outcomes in individuals with non–central nervous system cancers who received various types of treatments (Amidi & Wu, 2019). Throughout most of the studies, structural brain changes were identified following cancer treatments that included evidence of reduced global and local gray matter volumes, impairments in white matter microstructural integrity, and brain network alterations.
Oxidative stress occurs because of an imbalance between reactive oxygen species and antioxidants, and is implicated as a mechanism for CRCI. One review focused on an examination of the effects of oxidative stress on CRCI in preclinical and clinical studies of chemotherapy administration (Cauli, 2021). Findings suggest that oxidative stress contributes to CRCI by causing changes in the expression and activity of pro- and antioxidant enzymes, changes to signal transduction pathways, DNA and RNA damage, and regulation of gene expression (Cauli, 2021). As noted by Sordillo and Sordillo (2020), chemotherapy can lead to the production of reactive oxygen species in the brain, which results in increased emissions of biophotons that may contribute to neuronal pathology.
Three studies evaluated for associations between neurofilament proteins (i.e., biomarkers of axonal damage) and CRCI (Argyriou et al., 2021; Liu et al., 2020; Natori et al., 2015). In a study of women with breast cancer receiving chemotherapy (Natori et al., 2015), serum high molecular weight neurofilament subunit was evaluated as a predictive marker of CRCI. Although high molecular weight neurofilament subunit levels increased in a dose-dependent manner, no associations were found with changes in cognitive measures. In studies of patients with gastric (Liu et al., 2020) and breast cancers (Argyriou et al., 2021), no associations were found between neurofilament light chain levels and objective measures of CRCI.
Accelerated brain aging caused by cancer treatments is another potential mechanism for CRCI. In a longitudinal study of women with breast cancer (Henneghan, Rao, et al., 2020), a neuroimaging-based machine learning algorithm was used to predict brain age. Compared to healthy controls, findings suggest positive correlations between brain aging metrics and cognitive impairment (i.e., verbal memory interference), as well as acute decreases in cortical thickness.
Two studies evaluated for associations between biomarkers that may be reflective of accelerated biologic aging and CRCI. In a study of breast cancer survivors (Henneghan, Haley, & Kesler, 2020), prediction models were created and evaluated to predict objective cognitive performance using measures of amyloid beta 42, amyloid beta 40, tau, and 13 cytokines. Results suggest that neurodegenerative biomarkers interact with cytokines to influence the persistence of CRCI into survivorship. In another study of breast cancer survivors (Carroll et al., 2018), high leukocyte DNA damage and low telomerase activity were associated with worse executive function. In addition, high leukocyte DNA damage was associated with worse memory, and low telomerase activity was associated with worse attention and motor speed. Additional research is needed to understand how accelerated biologic aging may contribute to CRCI.
An emerging area of research is the evaluation of associations between DNA methylation and CRCI. For example, in a longitudinal study of patients with breast cancer (Yao et al., 2019), increased methylation at one cytosine-phosphate-guanine site (i.e., cg16936953) was associated with decrements in self-reported cognitive function. In another longitudinal study of patients with early-stage breast cancer (Yang et al., 2019), 56 differentially methylated positions were associated with decreases in objectively measured memory.
Gene expression studies provide information about cellular responses to environmental changes (Singh et al., 2018). Although studies of associations between CRCI and changes in gene expression are limited, they can be used to identify perturbed biologic pathways associated with CRCI. For example, in a study that evaluated differentially expressed genes and perturbed pathways between patients with cancer who did or did not report CRCI (Oppegaard et al., 2021), perturbations in cytokine-specific pathways, as well as pathways involved in cytokine production and cytokine activation, were identified.
In terms of autoimmune responses, one study evaluated neuronal autoantibodies associated with objective reports of CRCI in patients with melanoma (Bartels et al., 2019). Compared to patients who were antibody-negative, patients who were antibody-positive (i.e., immunoglobulin A, immunoglobulin M, and anti-N-methyl-D-aspartate receptor antibodies) were at increased risk for CRCI throughout multiple cognitive domains. Although research in oncology is limited, N-methyl-D-aspartate receptor antibodies are associated with other types of cognitive impairment (e.g., encephalitis, dementia) (Bartels et al., 2019).
Another emerging hypothesized mechanism for CRCI is alterations in the neuroprotective effects of a type of extracellular vesicle called exosomes. One review suggested that exosomes play a role in neuronal cell communication, have the ability to cross the blood–brain barrier, and have roles in neurodegeneration and neuroprotection (Koh et al., 2020). Future research focused on total or cell-type–specific exosomes may identify novel mechanisms for CRCI. Equally important, stem cell–derived exosomes may be useful as a therapeutic intervention for CRCI (Srivastava & Singh, 2020).
Disruptions of the microbiota-gut-brain (MGB) axis may be another mechanism for CRCI (Ciernikova et al., 2021; Jordan et al., 2018; Song & Bai, 2021; Subramaniam et al., 2020). The MGB axis represents a bidirectional communication pathway between the gastrointestinal tract and the brain (Davidson et al., 2018). Microbiota–brain communication is facilitated through microbial metabolites (e.g., neurotransmitters, short-chain fatty acids) (Subramaniam et al., 2020). Chemotherapy-induced nausea was associated with memory problems as well as other symptoms (e.g., fatigue, mood swings) that may be linked to alterations in the MGB axis (Song & Bai, 2021).
Taken together, many plausible biologic mechanisms for CRCI exist. In addition, as noted in Table 1, many important mechanistic-based questions warrant investigation. Importantly, future studies can use the MMCRCI to ensure that mechanism-focused studies include evaluations of other important factors.
Implications for Nursing
The MMCRCI has numerous implications for nursing practice and research. Nurses are the clinicians who interact most with patients throughout the cancer care continuum. Nurses can assess patients for cognitive changes and provide education, support, and referrals. Knowledge of the occurrence of CRCI and factors that contribute to this symptom will allow for better assessments of modifiable and nonmodifiable risk factors. Using the MMCRCI, nurses can identify patients who may want to participate in research studies.
Importantly, the MMCRCI highlights the need for studies that evaluate CRCI in the context of its multiple contributing factors. Nurse scientists can use this model to design future studies that take a more comprehensive approach to understanding CRCI. In doing so, effective interventions to prevent and treat this symptom will be identified.
Conclusion
Based on several decades of research, CRCI knowledge has increased substantially. However, in addition to the conceptual and empirical issues, a comprehensive conceptual model of CRCI is lacking. Therefore, the MMCRCI was developed to summarize existing knowledge and provide a framework to guide additional research. As with other symptoms (e.g., fatigue), the National Cancer Institute, in collaboration with professional organizations (e.g., Oncology Nursing Society, American Society of Clinical Oncology, Multinational Association of Supportive Care in Cancer, International Cognition and Cancer Task Force), needs to convene a state-of-the-science conference to develop a consensus on the definition of CRCI, preferred methods to assess CRCI, and directions for additional research.
Given the effects of CRCI on patients receiving treatments for cancer and survivors, other omics approaches (e.g., genomics, transcriptomics) as well as electroencephalographic measurements of brain activity (e.g., frontal-midline theta), will be explored as potential CRCI indicators or used to evaluate the efficacy of CRCI interventions. Although it would be ideal to evaluate all the concepts and components of the MMCRCI in a comprehensive fashion, this approach may be cost prohibitive. However, investigators with existing datasets can evaluate portions of the model to determine directionality for some of the proposed relationships. In addition, the MMCRCI can be used to design preclinical and clinical studies of CRCI. As more research is conducted, the MMCRCI will need to be updated or refined.

About the Authors
Kate R. Oppegaard, RN, MS, OCN®, is a PhD student in the Department of Physiological Nursing at the University of California, San Francisco; Samantha J. Mayo, RN, PhD, is an associate professor in the Lawrence S. Bloomberg Faculty of Nursing at the University of Toronto in Canada; Terri S. Armstrong, PhD, RN, ANP-BC, FAANP, FAAN, is a senior investigator in the Neuro-Oncology Branch in the National Cancer Institute of the National Institutes of Health in Bethesda, MD; and Joaquin A. Anguera, PhD, is an associate professor in the Department of Neurology and Psychiatry, Kord M. Kober, PhD, is an associate professor in the Department of Physiological Nursing, and Christine Miaskowski, RN, PhD, is a professor in the Department of Physiological Nursing and the Department of Anesthesia and Perioperative Care, all at the University of California, San Francisco. This research was supported by grants from the Oncology Nursing Foundation and the National Institute of Nursing Research of the National Institutes of Health (T32NR016920). All authors contributed to the conceptualization and design. Oppegaard completed the data collection. All authors contributed to the manuscript preparation. Oppegaard can be reached at kate.oppegaard@ucsf.edu, with copy to ONFEditor@ons.org. (Submitted April 2022. Accepted September 23, 2022.)
References
Ahles, T.A., & Hurria, A. (2018). New challenges in psycho-oncology research IV: Cognition and cancer: Conceptual and methodological issues and future directions. Psycho-Oncology, 27(1), 3–9. https://doi.org/10.1002/pon.4564
Ahles, T.A., & Root, J.C. (2018). Cognitive effects of cancer and cancer treatments. Annual Review of Clinical Psychology, 14, 425–451. https://doi.org/10.1146/annurev-clinpsy-050817-084903
Alonso-Lana, S., Marquié, M., Ruiz, A., & Boada, M. (2020). Cognitive and neuropsychiatric manifestations of COVID-19 and effects on elderly individuals with dementia. Frontiers in Aging Neuroscience, 12. https://doi.org/10.3389/fnagi.2020.588872
Amidi, A., & Wu, L.M. (2019). Structural brain alterations following adult non-CNS cancers: A systematic review of the neuroimaging literature. Acta Oncologica, 58(5), 522–536. https://doi.org/10.1080/0284186x.2018.1563716
Andreotti, C., Root, J.C., Ahles, T.A., McEwen, B.S., & Compas, B.E. (2015). Cancer, coping, and cognition: A model for the role of stress reactivity in cancer-related cognitive decline. Psycho-Oncology, 24(6), 617–623. https://doi.org/10.1002/pon.3683
Argyriou, A.A., Karteri, S., Bruna, J., Mariotto, S., Simo, M., Velissaris, D., . . . Kalofonos, H.P. (2021). Serum neurofilament light chain levels as biomarker of paclitaxel-induced cognitive impairment in patients with breast cancer: A prospective study. Supportive Care in Cancer, 30(2), 1807–1814. https://doi.org/10.1007/s00520-021-06509-x
Arndt, J., Das, E., Schagen, S.B., Reid-Arndt, S.A., Cameron, L.D., & Ahles, T.A. (2013). Broadening the cancer and cognition landscape: The role of self-regulatory challenges. Psycho-Oncology, 23(1), 1–8. https://doi.org/10.1002/pon.3351
Bai, L., & Yu, E. (2021). A narrative review of risk factors and interventions for cancer-related cognitive impairment. Annual Translation of Medicine, 9(1), 72. https://doi.org/10.21037/atm-20-6443
Bartels, F., Strönisch, T., Farmer, K., Rentzsch, K., Kiecker, F., & Finke, C. (2019). Neuronal autoantibodies associated with cognitive impairment in melanoma patients. Annals of Oncology, 30(5), 823–829. https://doi.org/10.1093/annonc/mdz083
Bernstein, L.J., McCreath, G.A., Komeylian, Z., & Rich, J.B. (2017). Cognitive impairment in breast cancer survivors treated with chemotherapy depends on control group type and cognitive domains assessed: A multilevel meta-analysis. Neuroscience and Biobehavioral Review, 83, 417–428. https://doi.org/10.1016/j.neubiorev.2017.10.028
Boykoff, N., Moieni, M., & Subramanian, S.K. (2009). Confronting chemobrain: An in-depth look at survivors’ reports of impact on work, social networks, and health care response. Journal of Cancer Survivorship, 3(4), 223. https://doi.org/10.1007/s11764-009-0098-x
Buskbjerg, C.D.R., Amidi, A., Demontis, D., Nissen, E.R., & Zachariae, R. (2019). Genetic risk factors for cancer-related cognitive impairment: A systematic review. Acta Oncologica, 58(5), 537–547. https://doi.org/10.1080/0284186X.2019.1578410
Campbell, K.L., Zadravec, K., Bland, K.A., Chesley, E., Wolf, F., & Janelsins, M.C. (2020). The effect of exercise on cancer-related cognitive impairment and applications for physical therapy: Systematic review of randomized controlled trials. Physical Therapy, 100(3), 523–542. https://doi.org/10.1093/ptj/pzz090
Carroll, J.E., Van Dyk, K., Bower, J.E., Scuric, Z., Petersen, L., Schiestl, R., . . . Ganz, P.A. (2018). Cognitive performance in survivors of breast cancer and markers of biologic aging. Cancer, 125(2), 298–306. https://doi.org/10.1002/cncr.31777
Castel, H., Denouel, A., Lange, M., Tonon, M.-C., Dubois, M., & Joly, F. (2017). Biomarkers associated with cognitive impairment in treated cancer patients: Potential predisposition and risk factors. Frontiers in Pharmacology, 8, 138–138. https://doi.org/10.3389/fphar.2017.00138
Cauli, O. (2021). Oxidative stress and cognitive alterations induced by cancer chemotherapy drugs: A scoping review. Antioxidants, 10(7), 1116. https://doi.org/10.3390/antiox10071116
Chia, Z., Parks, R.M., & Cheung, K.-L. (2021). Does breast cancer surgery impact functional status and independence in older patients? A narrative review. Oncology Therapy, 9(2), 373–383. https://doi.org/10.1007/s40487-021-00170-4
Chinn, P.L., & Kramer, M.K. (2011). Integrated theory and knowledge development in nursing (8th ed.). Elsevier.
Ciernikova, S., Mego, M., & Chovanec, M. (2021). Exploring the potential role of the gut microbiome in chemotherapy-induced neurocognitive disorders and cardiovascular toxicity. Cancers, 13(4), 782. https://doi.org/10.3390/cancers13040782
Coughlin, S.S. (2019). Social determinants of breast cancer risk, stage, and survival. Breast Cancer Research and Treatments, 177(3), 537–548. https://doi.org/10.1007/s10549-019-05340-7
Coughlin, S.S. (2020). Social determinants of colorectal cancer risk, stage, and survival: A systematic review. International Journal of Colorectal Disease, 35(6), 985–995. https://doi.org/10.1007/s00384-020-03585-z
Cui, B., Peng, F., Lu, J., He, B., Su, Q., Luo, H., . . . Liu, Q. (2021). Cancer and stress: NextGen strategies. Brain, Behavior, and Immunity, 93, 368–383. https://doi.org/10.1016/j.bbi.2020.11.005
Curtis, R.G., Windsor, T.D., & Soubelet, A. (2015). The relationship between big-5 personality traits and cognitive ability in older adults—A review. Aging, Neuropsychology, and Cognition, 22(1), 42–71. https://doi.org/10.1080/13825585.2014.888392
Davidson, G.L., Cooke, A.C., Johnson, C.N., & Quinn, J.L. (2018). The gut microbiome as a driver of individual variation in cognition and functional behaviour. Philosophical Transactions of the Royal Society B: Biological Sciences, 373(1756). https://doi.org/10.1098/rstb.2017.0286
Dijkshoorn, A.B.C., van Stralen, H.E., Sloots, M., Schagen, S.B., Visser-Meily, J.M.A., & Schepers, V.P.M. (2021). Prevalence of cognitive impairment and change in patients with breast cancer: A systematic review of longitudinal studies. Psycho-Oncology, 30(5), 635–648. https://doi.org/10.1002/pon.5623
Du, J., Zhang, A., Li, J., Liu, X., Wu, S., Wang, B., . . . Jia, H. (2021). Doxorubicin-induced cognitive impairment: The mechanistic insights. Frontiers in Oncology, 11. https://doi.org/10.3389/fonc.2021.673340
Earp, J.A., & Ennett, S.T. (1991). Conceptual models for health education research and practice. Health Education Research, 6(2), 163–171. https://doi.org/10.1093/her/6.2.163
Eide, S., & Feng, Z.-P. (2020). Doxorubicin chemotherapy-induced “chemo-brain”: Meta-analysis. European Journal of Pharmacology, 881, 173078. https://doi.org/10.1016/j.ejphar.2020.173078
El-Agamy, S.E., Abdel-Aziz, A.K., Esmat, A., & Azab, S.S. (2019). Chemotherapy and cognition: Comprehensive review on doxorubicin-induced chemobrain. Cancer Chemotherapy and Pharmacology, 84(1), 1–14. https://doi.org/10.1007/s00280-019-03827-0
Evans, I.E.M., Llewellyn, D.J., Matthews, F.E., Woods, R.T., Brayne, C., & Clare, L. (2018). Social isolation, cognitive reserve, and cognition in healthy older people. PLOS ONE, 13(8), e0201008. https://doi.org/10.1371/journal.pone.0201008
Farahani, M.A., Soleimanpour, S., Mayo, S.J., Myers, J.S., Panesar, P., & Ameri, F. (2022). The effect of mind-body exercise on cognitive function in cancer survivors: A systematic review. Canadian Oncology Nursing Journal, 32(1), 38–48.
Figueroa, J.F., Frakt, A.B., & Jha, A.K. (2020). Addressing social determinants of health: Time for a polysocial risk score. JAMA, 323(16), 1553–1554. https://doi.org/10.1001/jama.2020.2436
Fletcher, D., & Sarkar, M. (2013). Psychological resilience: A review and critique of definitions, concepts, and theory. European Psychologist, 18(1), 12–23. https://doi.org/10.1027/1016-9040/a000124
García-Sánchez, J., Torregrosa, M.D., & Cauli, O. (2020). Cognitive functions under anti-HER2 targeted therapy in cancer patients: A scoping review. Endocrine, Metabolic and Immune Disorders—Drug Targets, 21(7), 1163–1170. http://doi.org/10.2174/1871530320666200729153009
Harrison, R.A., Sharafeldin, N., Rexer, J.L., Streck, B., Petersen, M., Henneghan, A.M., & Kesler, S.R. (2021). Neurocognitive impairment after hematopoietic stem cell transplant for hematologic malignancies: Phenotype and mechanisms. Oncologist, 26(11), e2021–e2033. https://doi.org/10.1002/onco.13867
Hedayati, E., Alinaghizadeh, H., Schedin, A., Nyman, H., & Albertsson, M. (2012). Effects of adjuvant treatment on cognitive function in women with early breast cancer. European Journal of Oncology Nursing, 16(3), 315–322. https://doi.org/10.1016/j.ejon.2011.07.006
Henneghan, A. (2015). Modifiable factors and cognitive dysfunction in breast cancer survivors: A mixed-method systematic review. Supportive Care in Cancer, 24(1), 481–497. https://doi.org/10.1007/s00520-015-2927-y
Henneghan, A., Haley, A.P., & Kesler, S. (2020). Exploring relationships among peripheral amyloid beta, tau, cytokines, cognitive function, and psychosomatic symptoms in breast cancer survivors. Biologic Research in Nursing, 22(1), 126–138.
Henneghan, A., Rao, V., Harrison, R.A., Karuturi, M., Blayney, D.W., Palesh, O., & Kesler, S.R. (2020). Cortical brain age from pre-treatment to post-chemotherapy in patients with breast cancer. Neurotoxicity Research, 37(4), 788–799. https://doi.org/10.1007/s12640-019-00158-z
Hermelink, K., Küchenhoff, H., Untch, M., Bauerfeind, I., Lux, M.P., Bühner, M., . . . Münzel, K. (2010). Two different sides of ‘chemobrain’: Determinants and nondeterminants of self-perceived cognitive dysfunction in a prospective, randomized, multicenter study. Psycho-Oncology, 19(12), 1321–1328. https://doi.org/10.1002/pon.1695
Hess, L.M., & Insel, K.C. (2007). Chemotherapy-related change in cognitive function: A conceptual model. Oncology Nursing Forum, 34(5), 981–994. https://doi.org/10.1188/07.ONF.981-994
Horowitz, T.S., Suls, J., & Treviño, M. (2018). A call for a neuroscience approach to cancer-related cognitive impairment. Trends in Neurosciences, 41(8), 493–496. https://doi.org/10.1016/j.tins.2018.05.001
Hurt, C.S., Landau, S., Burn, D.J., Hindle, J.V., Samuel, M., Wilson, K., & Brown, R.G. (2012). Cognition, coping, and outcome in Parkinson’s disease. International Psychogeriatrics, 24(10), 1656–1663. https://doi.org/10.1017/S1041610212000749
Hwang, S.Y., Kim, K., Ha, B., Lee, D., Kim, S., Ryu, S., . . . Jung, S.J. (2021). Neurocognitive effects of chemotherapy for colorectal cancer: A systematic review and a meta-analysis of 11 studies. Cancer Research and Treatment, 53(4), 1134–1147. https://doi.org/10.4143/crt.2020.1191
Jacobsen, P.B., Garland, L.L., Booth-Jones, M., Donovan, K.A., Thors, C.L., Winters, E., & Grendys, E. (2004). Relationship of hemoglobin levels to fatigue and cognitive functioning among cancer patients receiving chemotherapy. Journal of Pain and Symptom Management, 28(1), 7–18. https://doi.org/10.1016/j.jpainsymman.2003.11.002
Janelsins, M.C., Kesler, S.R., Ahles, T.A., & Morrow, G.R. (2014). Prevalence, mechanisms, and management of cancer-related cognitive impairment. International Review of Psychiatry, 26(1), 102–113. https://doi.org/10.3109/09540261.2013.864260
Joly, F., Castel, H., Tron, L., Lange, M., & Vardy, J. (2019). Potential effect of immunotherapy agents on cognitive function in cancer patients. Journal of the National Cancer Institute, 112(2), 123–127. https://doi.org/10.1093/jnci/djz168
Jordan, K.R., Loman, B.R., Bailey, M.T., & Pyter, L.M. (2018). Gut microbiota-immune-brain interactions in chemotherapy-associated behavioral comorbidities. Cancer, 124(20), 3990–3999. https://doi.org/10.1002/cncr.31584
Kanaskie, M.L. (2012). Chemotherapy-related cognitive change: A principle-based concept analysis. Oncology Nursing Forum, 39(3), E241–E248. https://doi.org/10.1188/12.ONF.E241-E248
Kim, H.-J., Jung, S.-O., Kim, H., & Abraham, I. (2020). Systematic review of longitudinal studies on chemotherapy-associated subjective cognitive impairment in cancer patients. Psycho-Oncology, 29(4), 617–631. https://doi.org/10.1002/pon.5339
Klemencic, S., & Perkins, J. (2019). Diagnosis and management of oncologic emergencies. Western Journal of Emergency Medicine, 20(2), 316–322. https://doi.org/10.5811/wesjem.2018.12.37335
Koh, Y.Q., Tan, C.J., Toh, Y.L., Sze, S.K., Ho, H.K., Limoli, C.L., & Chan, A. (2020). Role of exosomes in cancer-related cognitive impairment. International Journal of Molecular Science, 21(8). https://doi.org/10.3390/ijms21082755
Lange, M., Joly, F., Vardy, J., Ahles, T., Dubois, M., Tron, L., . . . Castel, H. (2019). Cancer-related cognitive impairment: An update on state of the art, detection, and management strategies in cancer survivors. Annals of Oncology, 30(12), 1925–1940. https://doi.org/10.1093/annonc/mdz410
Lange, M., Licaj, I., Clarisse, B., Humbert, X., Grellard, J.-M., Tron, L., & Joly, F. (2019). Cognitive complaints in cancer survivors and expectations for support: Results from a web-based survey. Cancer Medicine, 8(5), 2654–2663. https://doi.org/10.1002/cam4.2069
Lara, E., Caballero, F.F., Rico-Uribe, L.A., Olaya, B., Haro, J.M., Ayuso-Mateos, J.L., & Miret, M. (2019). Are loneliness and social isolation associated with cognitive decline? International Journal of Geriatric Psychiatry, 34(11), 1613–1622. https://doi.org/10.1002/gps.5174
Liu, S., Huang, Z., Zhang, L., Pan, J., Lei, Q., Meng, Y., & Li, Z. (2020). Plasma neurofilament light chain may be a biomarker for the inverse association between cancers and neurodegenerative diseases. Frontiers in Aging Neuroscience, 12, 10. https://doi.org/10.3389/fnagi.2020.00010
Majoka, M.A., & Schimming, C. (2021). Effect of social determinants of health on cognition and risk of Alzheimer disease and related dementias. Clinical Therapeutics, 43(6), 922–929. https://doi.org/10.1016/j.clinthera.2021.05.005
Mampay, M., Flint, M.S., & Sheridan, G.K. (2021). Tumour brain: Pretreatment cognitive and affective disorders caused by peripheral cancers. British Journal of Pharmacology, 178(19), 3977–3996. https://doi.org/10.1111/bph.15571
Mayo, S.J., Lustberg, M., Dhillon, H.M., Nakamura, Z.M., Allen, D.H., Von Ah, D.M.C.J., . . . Peters, K.B. (2021). Cancer-related cognitive impairment in patients with non-central nervous system malignancies: An overview for oncology providers from the MASCC Neurological Complications Study Group. Supportive Care in Cancer, 29(6), 2821–2840. https://doi.org/10.1007/s00520-020-05860-9
Miaskowski, C., Cooper, B.A., Melisko, M., Chen, L.M., Mastick, J., West, C., . . . Aouizerat, B.E. (2014). Disease and treatment characteristics do not predict symptom occurrence profiles in oncology outpatients receiving chemotherapy. Cancer, 120(15), 2371–2378. https://doi.org/10.1002/cncr.28699
Monje, M., Borniger, J.C., D’Silva, N.J., Deneen, B., Dirks, P.B., Fattahi, F., . . . Winkler, F. (2020). Roadmap for the emerging field of cancer neuroscience. Cell, 181(2), 219–222. https://doi.org/10.1016/j.cell.2020.03.034
Morgans, A.K., Renzulli, J., Jr., Olivier, K., & Shore, N.D. (2021). Risk of cognitive effects in comorbid patients with prostate cancer treated with androgen receptor inhibitors. Clinical Genitourinary Cancer, 19(5), 467.e1–467.e411. https://doi.org/10.1016/j.clgc.2021.03.014
Myers, J.S. (2009). A comparison of the theory of unpleasant symptoms and the conceptual model of chemotherapy-related changes in cognitive function. Oncology Nursing Forum, 36(1), E1–E10. https://doi.org/10.1188/09.ONF.E1-E10
Myers, J.S., Erickson, K.I., Sereika, S.M., & Bender, C.M. (2018). Exercise as an intervention to mitigate decreased cognitive function from cancer and cancer treatment: An integrative review. Cancer Nursing, 41(4), 327–343. https://doi.org/10.1097/ncc.0000000000000549
Natori, A., Ogata, T., Sumitani, M., Kogure, T., Yamauchi, T., & Yamauchi, H. (2015). Potential role of pNF-H, a biomarker of axonal damage in the central nervous system, as a predictive marker of chemotherapy-induced cognitive impairment. Clinical Cancer Research, 21(6), 1348–1352. https://doi.org/10.1158/1078-0432.CCR-14-2775
Office of Disease Prevention and Health Promotion. (n.d.). Social determinants of health. Healthy People 2030. https://health.gov/healthypeople/objectives-and-data/social-determinant…
Olson, B., & Marks, D.L. (2019). Pretreatment cancer-related cognitive impairment—Mechanisms and outlook. Cancers, 11(5), 687. https://doi.org/10.3390/cancers11050687
Oppegaard, K., Harris, C.S., Shin, J., Paul, S.M., Cooper, B.A., Chan, A., . . . Kober, K.M. (2021). Cancer-related cognitive impairment is associated with perturbations in inflammatory pathways. Cytokine, 148, 155653. https://doi.org/10.1016/j.cyto.2021.155653
Potrata, B., Cavet, J., Blair, S., Howe, T., & Molassiotis, A. (2010). ‘Like a sieve’: An exploratory study on cognitive impairments in patients with multiple myeloma. European Journal of Cancer Care, 19(6), 721–728. https://doi.org/10.1111/j.1365-2354.2009.01145.x
Reid-Arndt, S.A., & Cox, C.R. (2012). Stress, coping and cognitive deficits in women after surgery for breast cancer. Journal of Clinical Psychology in Medical Settings, 19(2), 127–137. https://doi.org/10.1007/s10880-011-9274-z
Ren, X., Boriero, D., Chaiswing, L., Bondada, S., St. Clair, D.K., & Butterfield, D.A. (2019). Plausible biochemical mechanisms of chemotherapy-induced cognitive impairment (“chemobrain”), a condition that significantly impairs the quality of life of many cancer survivors. Biochimica et Biophysica Acta, 1865(6), 1088–1097. https://doi.org/10.1016/j.bbadis.2019.02.007
Rust, C., & Davis, C. (2013). Chemobrain in underserved African American breast cancer survivors: A qualitative study. Clinical Journal of Oncology Nursing, 17(2), E29–E34. https://doi.org/10.1188/13.CJON.E29-E34
Schaffrath, N., Oberste, M., & Zimmer, P. (2017). Effects of exercise interventions and physical activity behavior on cancer-related cognitive impairments: An update. Current Opinions in Supportive and Palliative Care, 11(1), 52–59. https://doi.org/10.1097/spc.0000000000000249
Schmidt, J.E., Beckjord, E., Bovbjerg, D.H., Low, C.A., Posluszny, D.M., Lowery, A.E., . . . Rechis, R. (2016). Prevalence of perceived cognitive dysfunction in survivors of a wide range of cancers: Results from the 2010 LIVESTRONG survey. Journal of Cancer Survivorship, 10(2), 302–311. https://doi.org/10.1007/s11764-015-0476-5
Shahid, M., & Kim, J. (2020). Exercise may affect metabolism in cancer-related cognitive impairment. Metabolites, 10(9), 377. https://doi.org/10.3390/metabo10090377
Singh, K.P., Miaskowski, C., Dhruva, A.A., Flowers, E., & Kober, K.M. (2018). Mechanisms and measurement of changes in gene expression. Biology Research in Nursing, 20(4), 369–382. https://doi.org/10.1177/1099800418772161
Song, B.C., & Bai, J. (2021). Microbiome-gut-brain axis in cancer treatment-related psychoneurological toxicities and symptoms: A systematic review. Supportive Care in Cancer, 29(2), 605–617. https://doi.org/10.1007/s00520-020-05739-9
Sordillo, P.P., & Sordillo, L.A. (2020). The mystery of chemotherapy brain: Kynurenines, tubulin and biophoton release. Anticancer Research, 40(3), 1189–1200. https://doi.org/10.21873/anticanres.14061
Sousa, H., Almeida, S., Bessa, J., & Pereira, M.G. (2020). The developmental trajectory of cancer-related cognitive impairment in breast cancer patients: A systematic review of longitudinal neuroimaging studies. Neuropsychology Review, 30(3), 287–309. https://doi.org/10.1007/s11065-020-09441-9
Southwick, S.M., Vythilingam, M., & Charney, D.S. (2005). The psychobiology of depression and resilience to stress: Implications for prevention and treatment. Annual Review of Clinical Psychology, 1, 255–291. https://doi.org/10.1146/annurev.clinpsy.1.102803.143948
Srivastava, R.K., & Singh, P. (2020). Stem cell therapies as a therapeutic option to counter chemo brain: A negative effect of cancer treatment. Regenerative Medicine, 15(6), 1789–1800. https://doi.org/10.2217/rme-2020-0060
Subramaniam, C.B., Bowen, J.M., Gladman, M.A., Lustberg, M.B., Mayo, S.J., & Wardill, H.R. (2020). The microbiota-gut-brain axis: An emerging therapeutic target in chemotherapy-induced cognitive impairment. Neuroscience and Biobehavioral Review, 116, 470–479. https://doi.org/10.1016/j.neubiorev.2020.07.002
Turnquist, C., Harris, B.T., & Harris, C.C. (2020). Radiation-induced brain injury: Current concepts and therapeutic strategies targeting neuroinflammation. Neuro-Oncology Advancements, 2(1). https://doi.org/10.1093/noajnl/vdaa057
Vardy, J., Dhillon, H.M., Pond, G.R., Rourke, S.B., Xu, W., Dodd, A., . . . Tannock, I.F. (2014). Cognitive function and fatigue after diagnosis of colorectal cancer. Annuals Oncology, 25(12), 2404–2412. https://doi.org/10.1093/annonc/mdu448
Vearncombe, K.J., Rolfe, M., Wright, M., Pachana, N.A., Andrew, B., & Beadle, G. (2009). Predictors of cognitive decline after chemotherapy in breast cancer patients. Journal of the International Neuropsychological Society, 15(6), 951–962.
Wefel, J.S., Kesler, S.R., Noll, K.R., & Schagen, S.B. (2014). Clinical characteristics, pathophysiology, and management of noncentral nervous system cancer-related cognitive impairment in adults. CA: A Cancer Journal for Clinicians, 65(2), 123–138. https://doi.org/10.3322/caac.21258
Wefel, J.S., Vardy, J., Ahles, T., & Schagen, S.B. (2011). International Cognition and Cancer Task Force recommendations to harmonise studies of cognitive function in patients with cancer. Lancet Oncology, 12(7), 703–708. https://doi.org/10.1016/s1470-2045(10)70294-1
Yang, G.S., Mi, X., Jackson-Cook, C.K., Starkweather, A.R., Lynch Kelly, D., Archer, K.J., . . . Lyon, D.E. (2019). Differential DNA methylation following chemotherapy for breast cancer is associated with lack of memory improvement at one year. Epigenetics, 15(5), 499–510. https://doi.org/10.1080/15592294.2019.1699695
Yang, Y., & Hendrix, C.C. (2018). Cancer-related cognitive impairment in breast cancer patients: Influences of psychological variables. Asia-Pacific Journal of Oncology Nursing, 5(3), 296–306. https://doi.org/10.4103/apjon.apjon_16_18
Yao, S., Hu, Q., Kerns, S., Yan, L., Onitilo, A.A., Misleh, J., . . . Janelsins, M.C. (2019). Impact of chemotherapy for breast cancer on leukocyte DNA methylation landscape and cognitive function: A prospective study. Clinical Epigenetics, 11(1), 45. https://doi.org/10.1186/s13148-019-0641-1
Zimmer, P., Baumann, F.T., Oberste, M., Wright, P., Garthe, A., Schenk, A., . . . Wolf, F. (2016). Effects of exercise interventions and physical activity behavior on cancer related cognitive impairments: A systematic review. BioMed Research International, 2016, 1–13. https://doi.org/10.1155/2016/1820954




