Glycemic Variability in Patients With Stage II–III Colon Cancer Treated With Surgery and Adjuvant Chemotherapy
Objectives: To examine glycemic variability within one month and one year following surgery and throughout adjuvant chemotherapy among patients with stage II–III colon cancer, with and without type 2 diabetes (T2D).
Sample & Setting: 58 patients with stage II–III colon cancer treated with surgery and chemotherapy.
Methods & Variables: A retrospective analysis of electronic health record data over one year showed glycemic variability, measured as standard deviation and coefficient of variation. Chi-square, Fisher’s exact, and Mann–Whitney U tests and Spearman’s correlation coefficient were calculated.
Results: Patients with T2D had higher glycemic variability throughout chemotherapy and within one year following surgery. A significant increase in glycemic variability throughout chemotherapy was observed in patients without T2D. Significant associations between glycemic variability and demographic and clinical characteristics differed by T2D status, standard deviation, and coefficient of variation.
Implications for Nursing: Nurses need to assess serial blood glucose levels in patients with and without T2D. Teaching patients how to maintain glycemic control during treatment is a priority. Research should include predictive models to identify risk factors for higher glycemic variability and cancer-related symptoms and outcomes.
Jump to a section
Patients with cancer, with and without type 2 diabetes (T2D), are known to experience hyperglycemia, which has been associated with poor cancer-related outcomes (Hammer et al., 2019; Healy et al., 2017; Simon et al., 2018; Zylla et al., 2019). Therefore, glycemic control is essential for improving outcomes in patients with cancer. The incidence of T2D in the U.S. general population is 10.5% (Centers for Disease Control and Prevention, 2020), yet diabetes (types 1 and 2) have been reported to occur in 21.94% of patients with early-stage (stage I–II) colon cancer (Lee et al., 2020). Importantly, prior to a cancer diagnosis, patients with T2D have a 27% higher risk of developing colon cancer, the fourth most diagnosed cancer in the United States (American Cancer Society, 2021), compared to individuals without T2D (González et al., 2017). In addition, patients with T2D and colorectal cancer have a 17% increased risk of all-cause mortality and a 12% increased risk of cancer-specific mortality (Mills et al., 2013). Of further concern, the rate of T2D in the United States is expected to rise from 35.6 million cases in 2015 to 54.9 million by 2030 (Rowley et al., 2017), and the rates of individuals with T2D and cancer will also increase.
There are no established, specific guidelines for glycemic management in patients with cancer with and without T2D. Hemoglobin A1c (HbA1c), the most commonly used assessment of glycemic status, provides a value that reflects a two- to three-month average of blood glucose (American Diabetes Association, 2021). However, HbA1c does not capture glycemic variability, described as fluctuations in blood glucose. In addition, HbA1c measures may be inaccurate in patients with cancer because of the malignancy, acute blood loss from surgery, and the apoptotic effect of chemotherapy on the life of erythrocytes (Campbell et al., 2019; Fayyaz et al., 2019; Gu et al., 2018). However, glycemic variability can be captured through repeated measures of blood glucose and reported as short-term (within-day or between-day) and long-term (visit-to-visit) variability for a more accurate assessment of glycemic control in patients with cancer (Monnier et al., 2018).
Glycemic variability occurs in patients with cancer with and without T2D; however, it has been reported to be higher among patients with T2D (Gude et al., 2017; Mandolfo et al., 2020, 2022; Suh & Kim, 2015). Of note, poor health outcomes have been associated with glycemic variability in hospitalized patients (Akirov et al., 2019; Atamna et al., 2019; Singh et al., 2018) and in patients with congestive heart failure (Gu et al., 2018), vascular complications (Gorst et al., 2015), diabetic neuropathy (Cheng et al., 2014; Lai et al., 2019), end-stage renal disease (Yang et al., 2015), and cognitive decline (Yu et al., 2019). Data are limited on the associations between glycemic variability and demographic characteristics. Demographic (e.g., older adults, non-White individuals) and clinical characteristics are known to influence outcomes in patients with T2D (American Diabetes Association, 2021; Hill-Briggs et al., 2021); however, studies that investigate social determinants of health, glycemic status, and cancer-related outcomes are lacking.
Although associations between glycemic status and cancer have been studied and reviewed for decades (Giovannucci et al., 2010; Hammer et al., 2019), few studies included the impact of glycemic variability on patient risks. From these limited studies, glycemic variability was found to be associated with increased risk of infection and non-relapse mortality in patients treated with allogeneic hematopoietic cell transplantation (with glycemic variability defined as standard deviation [SD] 29 mg/dl or greater) (Hammer et al., 2009), lower acute myelogenous leukemia remission rates (Kuhlman et al., 2019), and reduced survival in patients with acute myelogenous leukemia aged 60 years or older (Kuhlman et al., 2019). In addition, an increased risk for a cancer diagnosis in healthy individuals with and without T2D (Kobayashi et al., 2020; Saito et al., 2019) has been associated with glycemic variability.
Patients with colon cancer are an ideal population in which to study glycemic variability because of the known associations between glycemic instability and metabolic syndrome (Bowers et al., 2006), insulin resistance (Fujihara et al., 2012), and decreased diabetes medication adherence (Lund et al., 2021; Zanders et al., 2018). Particularly, patients with T2D and insulin resistance are at a higher risk of developing colon cancer (Giovannucci et al., 2010). Glycemic instability may occur from alterations in the sugar-sensing neuronal activity along the gastrointestinal tract (Fournel et al., 2016). Lastly, the complexity involved in colon cancer surgery and chemotherapy treatment may negatively affect a patient’s ability to self-manage T2D (Lund et al., 2021). Mandolfo et al. (2022) reported that patients with T2D experienced higher glycemic variability within one month to one year following surgery for colon cancer compared to patients without T2D. The authors did not find published reports of glycemic variability during adjuvant chemotherapy for the treatment of stage II–III colon cancer.
Objectives
The purpose of this study is to describe glycemic variability and factors associated with higher levels of glycemic variability in patients with colon cancer who were treated with adjuvant chemotherapy. The specific aims in a sample of patients with stage II–III colon cancer, with and without T2D, were to (a) determine levels of glycemic variability within and between groups; (b) describe associations between glycemic variability and preoperative glucose values and preoperative T2D; and (c) describe associations between glycemic variability and demographic and clinical characteristics.
Methods
Design
This was a retrospective, correlational analysis of electronic health record (EHR) data of patients with stage II–III colon cancer treated with surgery followed by chemotherapy. The conceptual model supporting this study was derived from the Malglycemia Orbit Model (Hammer & Voss, 2012). This original model describes the complex associations between malglycemia (hypoglycemia, hyperglycemia, and glycemic variability) (Hammer et al., 2009) and multiple factors in individuals with cancer. The Conceptual Model of Hyperglycemia in a Patient With Cancer offered additional support by describing the numerous biologic, physiologic, and intracellular pathways that contribute to and are affected by hyperglycemia in patients with cancer (Hammer et al., 2019).
Sample and Setting
All patients in the sample (N = 58) were treated at Nebraska Medicine, an 809-bed facility at the University of Nebraska Medical Center’s Fred and Pamela Buffett Cancer Center in Omaha. Patients diagnosed with stage II–III colon cancer between August 1, 2012, and November 30, 2018, were identified with data from the cancer center registry. Inclusion criteria were the following: aged older than 19 years, diagnosed with stage II–III colon cancer, record of administration of a 5-fluorouracil–based chemotherapy regimen, and two or more glucose values in the EHR within one year following colon surgery. Patients were excluded if they were being actively treated with chemotherapy for a malignancy other than colon cancer or if they had type 1 diabetes. The American Joint Committee on Cancer’s staging criteria were used to classify patients’ stage. Institutional review board approval was obtained from the University of Nebraska Medical Center.
Variables
The institution used Epic as the EHR. Table 1 displays all demographic and clinical characteristics, preoperative T2D status, glucose values, and cancer treatment information gathered. Only patients with a documented diagnosis of T2D or an International Classification of Diseases code (E08–E13) for T2D within their medical records were classified as having a diagnosis of T2D. All positive blood culture results were counted as bloodstream infections.
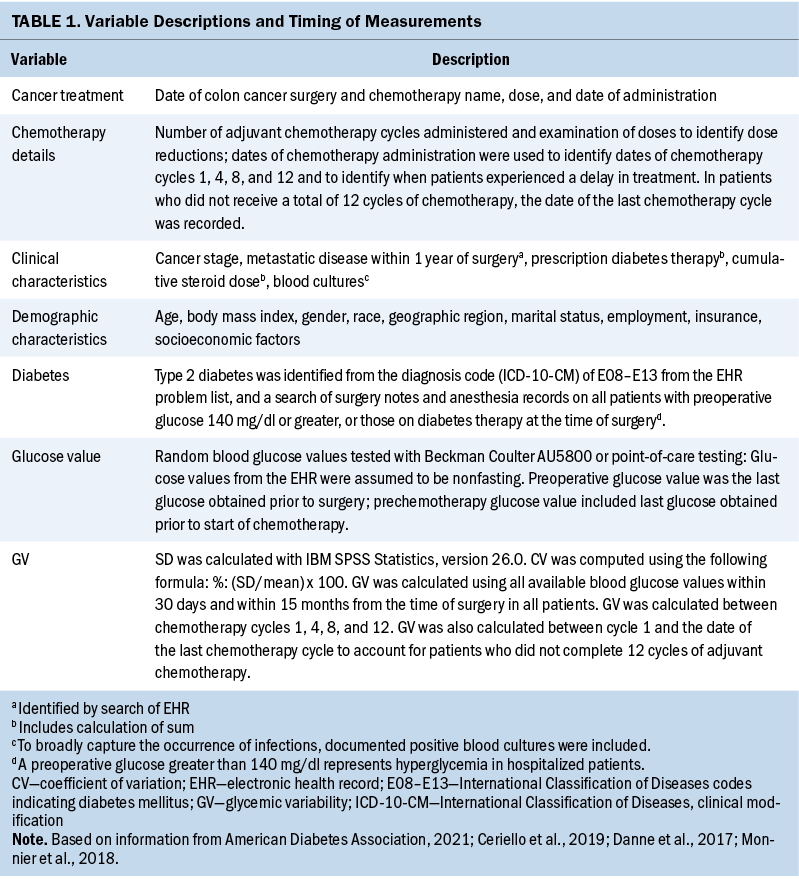
The outcome variable was glycemic variability, which was computed using all available glucose values extracted from the EHR and reported as SD (mg/dl) and coefficient of variation (CV) (%). SD computation was performed using IBM SPSS Statistics, version 26.0, and CV was calculated using the formula of percentage: %: (SD/mean) x 100 (Monnier et al., 2018).
Statistical Analysis
Data analyses were performed using IBM SPSS Statistics, version 26.0. Within- and between-group analyses were performed in groups of patients with and without T2D, and an assessment of normality was conducted using the Kolmogorov–Smirnov test. Continuous variables were presented as medians (interquartile range [IQR]). Categorical variables were presented by (N, %). Mann–Whitney U test was used to compare continuous variables. Chi-square test or Fisher’s exact test was used to compare categorical characteristics between groups of patients with and without T2D; results are reported as numbers and percentages. A Friedman test was used to examine a change in glycemic variability over time among the subset of patients who received 12 cycles of chemotherapy. Spearman’s correlation analyses were used to determine correlation coefficients between glycemic variability and a patient’s demographic and clinical characteristics. Statistical significance was set at p < 0.05.
Results
Baseline Demographic and Clinical Characteristics
All sample baseline demographic and clinical characteristics can be found in Tables 2 and 3. More than 80% of patients were White, with a mean age of 66 years in patients with T2D and 56 years in patients without T2D, and nearly all (96%) patients lived in the midwestern United States. Patients with T2D were significantly older than those without T2D (p = 0.025). The sample included patients with stage II–III colon cancer (N = 58) who were treated with surgery and chemotherapy. About 21% (n = 12) had a prior diagnosis of T2D. In total, 2,311 glucose values were retrieved.
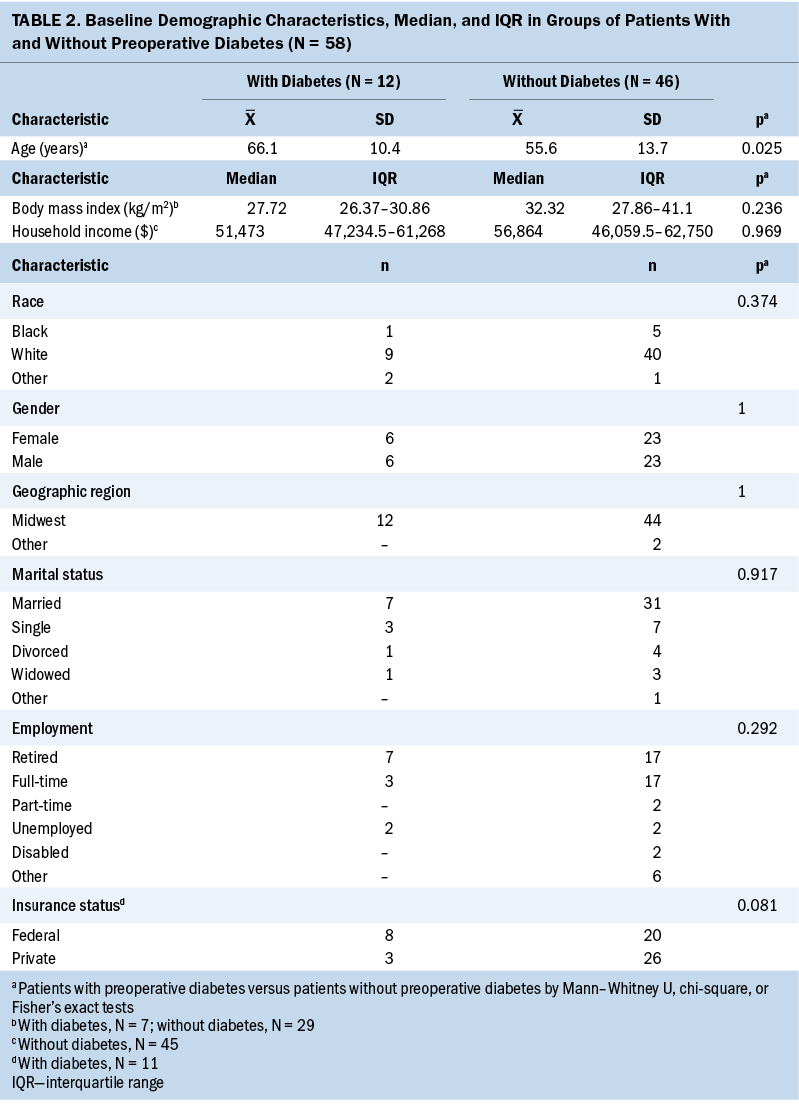
The preoperative glucose value was higher in patients with T2D compared to patients without T2D (p < 0.01). All patients received a 5-fluorouracil–based chemotherapy regimen with a median number of 12 cycles of adjuvant chemotherapy. T2D therapy at the time of surgery included insulin (25%, n = 3), oral therapy (25%, n = 3), or combined insulin and oral therapy (8.3%, n = 1). Five patients (41.7%) with diabetes had no record of T2D therapy.
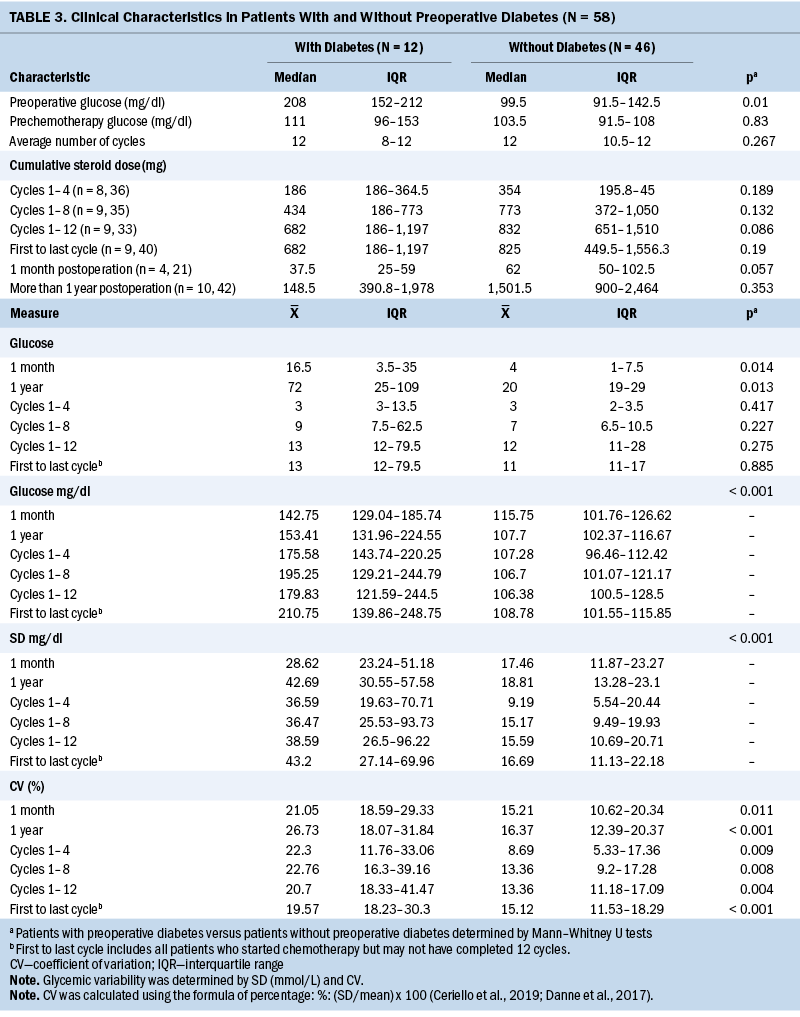
Glycemic Variability in Patients With and Without Type 2 Diabetes
Differences in the number of glucose measurements, mean glucose, SD, and CV between groups of patients with and without T2D within one month following surgery, throughout adjuvant chemotherapy, and within one year following surgery are shown in Table 4. Patients with T2D had 3.5 times more glucose measurements than those without T2D during the year following surgery (p = 0.013), and higher mean glucose, SD, and CV within one month following surgery. They also experienced higher mean glucose, SD, and CV within one year following surgery (p < 0.001). Higher mean glucoses were observed throughout adjuvant chemotherapy in patients with T2D between cycles 1 and 4, cycles 1 and 8, and cycles 1 and 12, and between the first and last cycles (p < 0.001). Patients with T2D also had significantly higher glycemic variability, as measured by SD and CV, throughout chemotherapy. Particularly, the SD was significantly higher in patients with T2D during chemotherapy between cycles 1 and 4, cycles 1 and 8, and cycles 1 and 12, and between the first and last cycles of chemotherapy (p < 0.001). CV was significantly higher in patients with T2D during chemotherapy between cycles 1 and 4 (p = 0.009), cycles 1 and 8 (p = 0.008), cycles 1 and 12 (20.7% versus 13.36%) (p = 0.004), and the first and last cycles (p < 0.001).
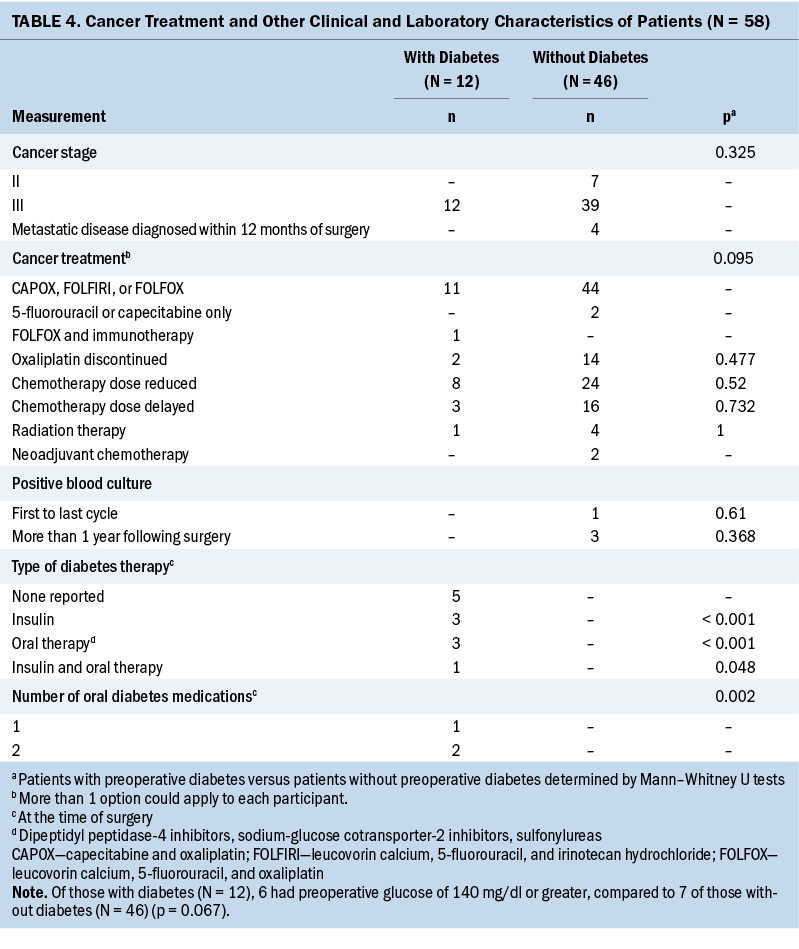
In patients with T2D (n = 8), there were no significant differences in SD and CV throughout 12 cycles of chemotherapy. A significant increase in glycemic variability, as measured by SD (p = 0 .013) and CV (p = 0.001) throughout 12 cycles of chemotherapy, was observed in patients without T2D (n = 21).
Glycemic Variability and Demographic and Clinical Characteristics
Glycemic variability and preoperative diabetes, preoperative glucose, and prechemotherapy glucose: Table 5 displays the Spearman’s correlation coefficient of the associations between glycemic variability as measured by SD and CV and preoperative T2D, preoperative glucose, and prechemotherapy glucose. Having a preoperative diagnosis of T2D was significantly associated with higher mean glucose and higher glycemic variability at every interval assessed during chemotherapy treatment. A higher preoperative blood glucose value was associated with a higher subsequent blood glucose value at one month following surgery, one year following surgery, and throughout adjuvant chemotherapy at cycles 1, 4, 8, and 12.
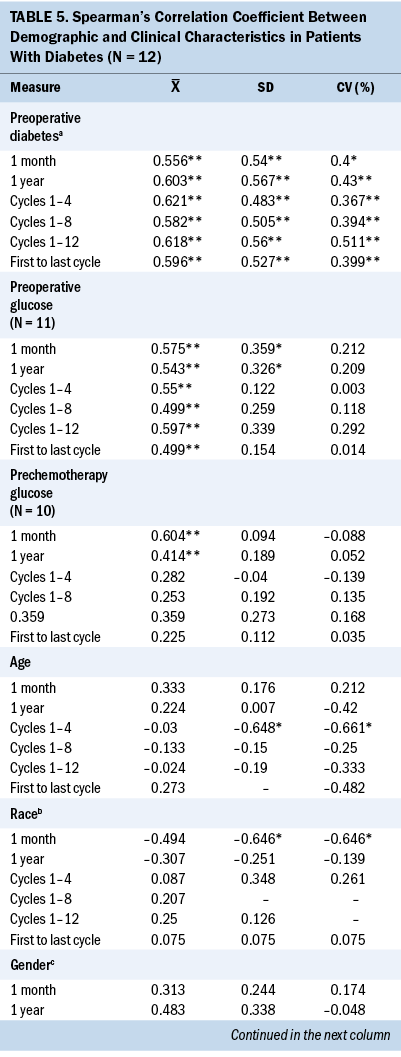
Patients With Type 2 Diabetes
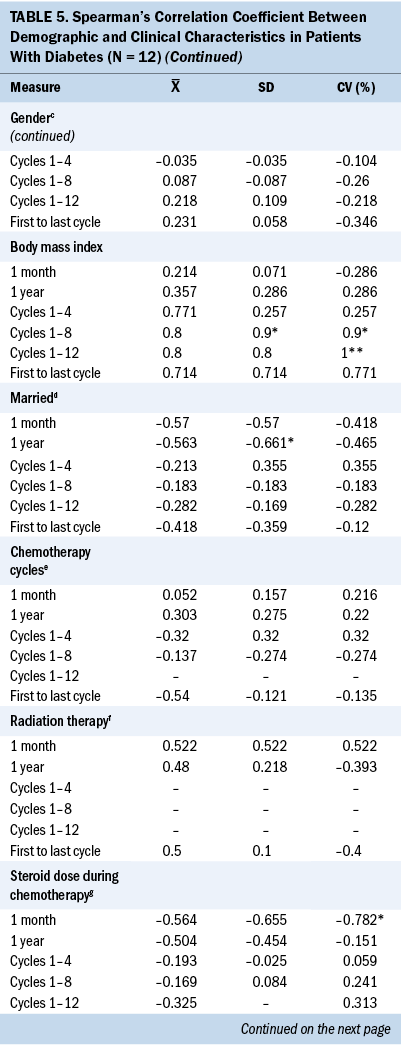
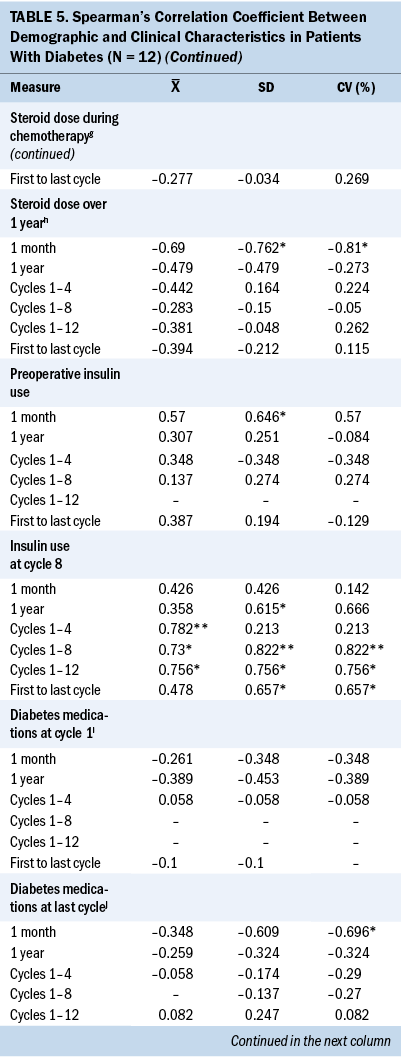
Significant associations were found between demographic characteristics and glycemic variability in patients with T2D. A younger age was significantly associated with higher SD (r = –0.648) and CV (r = –0.661) between cycles 1 and 4. Increased body mass index (BMI) was significantly associated with higher SD (r = 0.9) and CV (r = 0.9) between cycles 1 and 8 and 1 and 12 (r = 1). White patients were significantly associated with higher one-month SD (r = –0.646) and CV (r = –0.646), and married patients were significantly associated with higher one-year SD (r = –0.661).
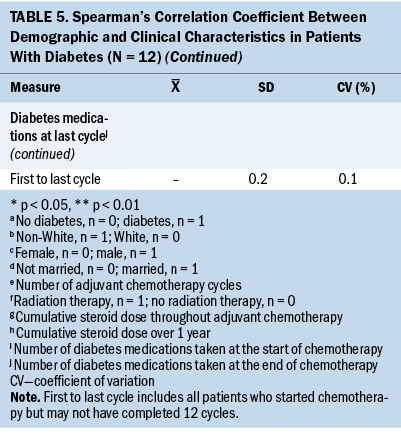
Significant associations were found between clinical characteristics and glycemic variability. Preoperative insulin use (r = 0.646) was significantly associated with higher one-month SD (r = 0.646). Insulin use at cycle 8 was associated with higher SD and CV between cycles 1 and 8 (r = 0.822, r = 0.822), between cycles 1 and 12 (r = 0.756, r = 0.756), between the first and last cycles of chemotherapy (r = 0.657, r = 0.657), and one-year SD (r = 0.615) and CV (r = 0.666). A higher one-month SD (r = –0.762) and one-month CV (r = –0.810) were associated with a lower cumulative steroid dose over one year. A higher one-month CV (r = –0.782) was associated with a lower cumulative steroid dose over the entire course (between the first and last cycles) of chemotherapy.
Patients Without Type 2 Diabetes
The only significant associations in patients without T2D were found between glycemic variability, as measured by SD and CV, and marital status. Not being married was associated with higher SD between the first and last cycles of chemotherapy (r = 0.323, p < 0.01), one-year SD (r = 0.396, p < 0.05), and one-year CV (r = 0.376, p < 0.05). No significant associations were noted between glycemic variability and all other demographic and clinical characteristics.
Discussion
To the authors’ knowledge, this was the first study to examine long-term glycemic variability throughout adjuvant chemotherapy and for one year following surgery in patients, with and without T2D, treated for stage II–III colon cancer. Not surprisingly, patients with T2D prior to surgery had higher preoperative glucose, preoperative hyperglycemia (glucose value of 140 mg/dl or greater) (American Diabetes Association, 2021), and higher prechemotherapy glucose compared to patients without T2D. Patients with T2D also had significantly higher glycemic variability throughout chemotherapy at each assessment. These findings are consistent with the literature, suggesting that patients with T2D have higher glycemic variability compared to those without T2D (Gude et al., 2017; Mandolfo et al., 2020, 2022; Suh & Kim, 2015). Patients without T2D experienced a significant change in glycemic variability over the course of 12 cycles of chemotherapy, despite experiencing overall lower glycemic variability compared to those with T2D. The findings from this study support the conceptual model, derived from the Malglycemia Orbit Model (Hammer & Voss, 2012), used in this study as it highlights the influence of demographic and clinical characteristics on glycemic variability in patients with cancer.
No specific guidelines exist for the management of glycemic control in patients with cancer. The patients with stage II–III colon cancer with T2D had high glycemic variability per the high cut-point values of 29 mg/dl or greater SD reported by Hammer et al. (2009), and very high upper limit IQR over the course of 12 cycles of chemotherapy. The median CV measurements throughout the course of chemotherapy did not exceed 36% in patients regardless of T2D status; however, there was high upper limit IQR between cycles 1 and 12 in patients with T2D. The measurement of SD or CV using serial blood glucoses can be used to assess glycemic control throughout adjuvant chemotherapy treatment (Ceriello et al., 2019; Kovatchev, 2019; Peyser et al., 2018). A benefit of using SD or CV is that blood glucose values are not affected by the life of the erythrocytes, unlike HbA1c (Campbell et al., 2019; Fayyaz et al., 2019). These findings support knowledge that glycemic variability and hyperglycemia, two critical components of malglycemia, represent two separate clinical factors (Ceriello et al., 2019; Hammer et al., 2009). Further research is needed to determine the metric for high glycemic variability, as measured by SD and CV, in patients with cancer and with and without T2D.
There are limited data of associations between glycemic variability and demographic and clinical characteristics in patients with colon cancer. This study identified that a younger age was strongly associated with higher SD and CV in patients with T2D between chemotherapy cycles 1 and 4. This finding differs from the positive association between age and glycemic variability noted in the general population (Gude et al., 2017). It is possible that the younger patients with T2D had other health conditions, such as obesity, that could have influenced glycemic variability in this study. Associations between increased glycemic variability and lower BMI have been reported in patients with and without T2D (Gude et al., 2017; Wang et al., 2017); however, this study identified that higher CV between chemotherapy cycles 1 and 8 and cycles 1 and 12 was strongly associated with higher BMI in patients with T2D. Few studies have evaluated associations between race and glycemic variability, and study findings have been inconsistent (Echouffo-Tcheugui et al., 2019; Hill et al., 2011; Kim et al., 2014). White patients were strongly associated with higher one-month glycemic variability, as measured by SD and CV, in patients with T2D. These results should be interpreted with caution because this study included few non-White patients.
This study found that 25% of patients with T2D were taking insulin, and another 25% were only taking oral diabetes therapy at the time of surgery. Insulin therapy is typically reserved for patients with uncontrolled T2D; therefore, the use of insulin at chemotherapy cycle 8 was strongly associated with glycemic variability between cycles 1 and 8 and cycles 1 and 12. Further studies are warranted evaluating the influence of insulin and oral diabetes medications on glycemic variability in patients with colon cancer who receive chemotherapy.
Steroid treatment, commonly prescribed as a chemotherapy premedication, results in hyperglycemia and increased risk for infections (Clement et al., 2004; Zylla et al., 2019). Higher one-month glycemic variability was associated with lower cumulative steroid dose throughout chemotherapy and over one year following surgery. This finding, although statistically significant, may not be clinically meaningful because there were inconsistencies in steroid use and the number of glucose measurements among patients. Further investigation is warranted. This study attempted to investigate the associations between positive blood cultures and glycemic variability, but no patients with T2D, and very few patients without T2D, experienced positive blood cultures within a year following surgery.
Strengths and Limitations
The use of the EHR provided an opportunity, although not prospectively designed, to collect information on many patients in an efficient, timely, cost-saving manner (Casey et al., 2016). The ability to evaluate 2,311 glucose values over one year in 58 patients treated with adjuvant chemotherapy for stage II–III colon cancer is a strength. The use of the EHR provided the opportunity to follow a patient’s trajectory throughout adjuvant chemotherapy treatment and within one year following surgery. All patients who received chemotherapy for stage II–III colon cancer were included in the sample, regardless of the number of cycles they completed. The small sample size of patients with T2D who completed 12 cycles of chemotherapy (n = 8) limited the study’s ability to identify significant changes in glycemic variability over time in patients with T2D.
The data sets were limited to patients from a single institution, and the majority of the patients were White and from the Midwest. Inherent threats of retrospective longitudinal studies were present, which may include selection bias regarding chemotherapy treatment decisions, inaccurate information in the EHR, missing data, and use of unmatched groups (Casey et al., 2016; Gordis, 2014). Despite searching to retrieve missing data from the EHR, the authors realize there may be patients with T2D or prediabetes who may not have been identified. Glucose values were assumed to be random, and the timing of meals in relation to the timing of the test was not captured in the EHR. In addition, the EHR problem list provided limited information because it does not include dates of diagnoses. Information on nutrition status was limited, and BMI was the only nutrition status indicator collected. Lastly, the data were not normally distributed, which limited the ability to perform multiple regression analyses and identify confounding variables. Statistical methods were limited to correlational analyses.
Implications for Nursing
Oncology nurses should be aware that, in the general population, patients with T2D have higher glycemic variability compared to those without T2D. This is important because glycemic variability is associated with complications, such as infection, lower remission rates, and mortality in patients with hematologic malignancies (Hammer et al., 2009, 2016; Kuhlman et al., 2019). These findings revealed that all patients with stage II–III colon cancer are at risk for increased glycemic variability throughout adjuvant chemotherapy. Oncology nurse clinicians should take the opportunity to discuss the importance of glycemic control for patients with colon cancer when providing prechemotherapy teaching. Additional topics of glycemic variability, nutrition, and self-management should be included in the assessment of patients diagnosed with T2D and colon cancer. Blood glucose values should be monitored, and glycemic variability should be calculated as a more complete assessment of glycemic control in patients receiving chemotherapy.
Research
Study findings provide insight and direction on glycemic variability in patients with colon cancer. Future research using predictive models to identify risk factors for higher glycemic variability and cancer-related symptoms and outcomes in a variety of solid tumor malignancies can contribute to the development of guidelines for improved glycemic control. Investigations of biomarkers, such as glycemic variability measures, in cancer symptom research is consistent with the Oncology Nursing Society’s research priority to study symptom science related to precision health and biomarkers (Von Ah et al., 2019).
Future studies of glycemic variability in patients with cancer should include investigating demographic and clinical characteristics that contribute to glycemic status. Assessment should include comorbidities, medications, and timing of glucose assessments in relation to meals. Studies should include associations between diabetes self-management and glycemic variability. Future research should consider the use of a continuous glucose monitor and time points for assessment of glycemic variability. Studies with larger samples, longer follow-up, and cohorts of patients with various solid tumor malignancies will lead to more robust investigations of associations between glycemic variability, tumor types, social determinants of health, healthcare use, and cancer-related outcomes.
Policy
The American College of Surgeons’ (2021) Commission on Cancer recommends on-site nutrition services by a registered dietitian as part of optimal cancer care. The authors advocate for cancer centers to offer diabetes self-management assessment to patients with T2D before cancer treatment. The American Nurses Association (2020) promotes the nurse’s role in care coordination, an essential part of oncology nursing. Oncology nurses should participate in the interprofessional management of glycemic control, support glycemic variability research, and advocate for policy change and development of glycemic variability guidelines in patients with and without T2D.
Conclusion
This study identified that patients with stage II–III colon cancer and preoperative T2D experienced higher glycemic variability within one month, throughout adjuvant chemotherapy, and within one year compared to those without T2D. Patients without T2D were vulnerable to significant increases in glycemic variability throughout chemotherapy. Associations between glycemic variability, measured by SD and CV, and demographic and clinical characteristics varied by preoperative T2D status and method of measurement. In patients with T2D, numerous strong associations between glycemic variability and demographic and clinical characteristics were observed. The measurement of glycemic variability in patients treated with surgery and chemotherapy for stage II–III colon cancer is feasible and provides another option for assessment of glycemic control when HbA1c may be unreliable.
About the Authors
Natalie Rasmussen Mandolfo, PhD, APRN-NP, AOCN®, was, at the time of this writing, a PhD student; Ann M. Berger, PhD, MSN, FAAN, is a professor and an advanced practice nurse, Leeza A. Struwe, PhD, MSN, RN, is an associate professor, and Marcia Y. Shade, BS, PhD, RN, is an assistant professor, all in the College of Nursing; Whitney Goldner, MD, is a physician and professor of medicine in the Division of Diabetes, Endocrinology, and Metabolism in the Department of Internal Medicine; Kelsey Klute, MD, is a medical oncologist and an assistant professor in the Division of Oncology and Hematology in the Department of Internal Medicine; and Sean J. Langenfeld, MD, FACS, FASCRS, is a professor of surgery in the Department of Surgery, all at the University of Nebraska Medical Center in Omaha; and Marilyn J. Hammer, PhD, DC, RN, FAAN, is the director of the Phyllis F. Cantor Center for Research in Nursing and Patient Care Services at the Dana-Farber Cancer Institute in Boston, MA. The research and publication were supported, in part, by the Fred and Pamela Buffett Cancer Center Support Grant from the National Cancer Institute under award number P30 CA036727 and the following scholarships: Oncology Nursing Society Foundation Research Doctoral Scholarship, Sigma Theta Tau International Gamma-Pi At Large Chapter, University of Nebraska Foundation Ann Malone Berger Ph.D. and Thomas Berger Nursing Fund, and University of Nebraska Foundation Versal H. and Edith Molly Stevens Caton Scholarship. Mandolfo, Berger, Shade, Goldner, Langenfeld, and Hammer contributed to the conceptualization and design. Mandolfo, Berger, and Struwe completed the data collection and provided statistical support. Mandolfo, Berger, Struwe, Goldner, and Langenfeld provided the analysis. All authors contributed to the manuscript preparation. Mandolfo can be reached at nmandolfo5@cox.net, with copy to ONFEditor@ons.org. (Submitted November 2021. Accepted March 21, 2022.)
References
Akirov, A., Shochat, T., Dotan, I., Diker-Cohen, T., Gorshtein, A., & Shimon, I. (2019). Glycemic variability and mortality in patients hospitalized in general surgery wards. Surgery, 166(2), 184–192. https://doi.org/10.1016/j.surg.2019.02.022
American Cancer Society. (2021). Cancer facts and figures, 2021. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and…
American College of Surgeons. (2021). Commission on Cancer: Optimal resources for cancer care: 2020 standards. https://www.facs.org/-/media/files/quality-programs/cancer/coc/optimal_…
American Diabetes Association. (2021). Standards of medical care in diabetes-2021. Diabetes Care, 44(Suppl. 1), S1–S2. https://care.diabetesjournals.org/content/44/Supplement_1
American Nurses Association. (2020). Health policy. https://www.nursingworld.org/practice-policy/health-policy
Atamna, A., Ayada, G., Akirov, A., Shochat, T., Bishara, J., & Elis, A. (2019). High blood glucose variability is associated with bacteremia and mortality in patients hospitalized with acute infection. QJM: Monthly Journal of the Association of Physicians, 112(2), 101–106. https://doi.org/10.1093/qjmed/hcy235
Bowers, K., Albanes, D., Limburg, P., Pietinen, P., Taylor, P.R., Virtamo, J., & Stolzenberg-Solomon, R. (2006). A prospective study of anthropometric and clinical measurements associated with insulin resistance syndrome and colorectal cancer in male smokers. American Journal of Epidemiology, 164(7), 652–664. https://doi.org/10.1093/aje/kwj253
Campbell, L., Pepper, T., & Shipman, K. (2019). HbA1c: A review of non-glycaemic variables. Journal of Clinical Pathology, 72(1), 12–19. https://doi.org/10.1136/jclinpath-2017-204755
Casey, J.A., Schwartz, B.S., Stewart, W.F., & Adler, N.E. (2016). Using electronic health records for population health research: A review of methods and applications. Annual Review of Public Health, 37(1), 61–81. https://doi.org/10.1146/annurev-publhealth-032315-021353
Centers for Disease Control and Prevention. (2020). National diabetes statistics report, 2020. https://www.cdc.gov/diabetes/data/statistics-report/index.html
Ceriello, A., Monnier, L., & Owens, D. (2019). Glycaemic variability in diabetes: Clinical and therapeutic implications. Lancet Diabetes and Endocrinology, 7(3), 221–230. https://doi.org/10.1016/S2213-8587(18)30136-0
Cheng, D., Fei, Y., Liu, Y., Li, J., Xue, Q., Wang, X., & Wang, N. (2014). HbA1C variability and the risk of renal status progression in diabetes mellitus: A meta-analysis. PLOS ONE, 9(12), e115509. https://doi.org/10.1371/journal.pone.0115509
Clement, S., Braithwaite, S.S., Magee, M.F., Ahmann, A., Smith, E.P., Schafer, R.G., & Hirsch, I.B. (2004). Management of diabetes and hyperglycemia in hospitals. Diabetes Care, 27(2), 553–591. https://doi.org/10.2337/diacare.27.2.553
Danne, T., Nimri, R., Battelino, T., Bergenstal, R.M., Close, K.L., DeVries, J.H., . . . Phillip, M. (2017). International consensus on use of continuous glucose monitoring. Diabetes Care, 40(12), 1631–1640. https://doi.org/10.2337/dc17-1600
Echouffo-Tcheugui, J.B., Zhao, S., Brock, G., Matsouaka, R.A., Kline, D., & Joseph, J.J. (2019). Visit-to-visit glycemic variability and risks of cardiovascular events and all-cause mortality: The ALLHAT study. Diabetes Care, 42(3), 486–493. https://doi.org/10.2337/dc18-1430
Fayyaz, B., Rehman, H.J., & Minn, H. (2019). Interpretation of hemoglobin A1C in primary care setting. Journal of Community Hospital Internal Medicine Perspectives, 9(1), 18–21. https://doi.org/10.1080/20009666.2018.1559432
Fournel, A., Marlin, A., Abot, A., Pasquio, C., Cirillo, C., Cani, P.D., & Knauf, C. (2016). Glucosensing in the gastrointestinal tract: Impact on glucose metabolism. American Journal of Physiology. Gastrointestinal and Liver Physiology, 310(9), G645–G658. https://doi.org/10.1152/ajpgi.00015.2016
Fujihara, S., Mori, H., Kobara, H., Nishiyama, N., Kobayashi, M., Oryu, M., & Masaki, T. (2012). Metabolic syndrome, obesity, and gastrointestinal cancer. Gastroenterology Research and Practice, 2012, 483623. https://doi.org/10.1155/2012/483623
Giovannucci, E., Harlan, D.M., Archer, M.C., Bergenstal, R.M., Gapstur, S.M., Habel, L.A., . . . Yee, D. (2010). Diabetes and cancer: A consensus report. Diabetes Care, 33(7), 1674–1685. https://doi.org/10.2337/dc10-0666
González, N., Prieto, I., Del Puerto-Nevado, L., Portal-Nuñez, S., Ardura, J.A., Corton, M., . . . Ortiz, A. (2017). 2017 update on the relationship between diabetes and colorectal cancer: Epidemiology, potential molecular mechanisms and therapeutic implications. Oncotarget, 8(11), 18456–18485. https://doi.org/10.18632/oncotarget.14472
Gordis, L. (2014). Epidemiology (5th ed.). Saunders.
Gorst, C., Kwok, C.S., Aslam, S., Buchan, I., Kontopantelis, E., Myint, P.K., . . . Mamas, M.A. (2015). Long-term glycemic variability and risk of adverse outcomes: A systematic review and meta-analysis. Diabetes Care, 38(12), 2354–2369. https://doi.org/10.2337/dc15-1188
Gu, J., Pan, J.A., Fan, Y.Q., Zhang, H.L., Zhang, J.F., & Wang, C.Q. (2018). Prognostic impact of HbA1c variability on long-term outcomes in patients with heart failure and type 2 diabetes mellitus. Cardiovascular Diabetology, 17, 96. https://doi.org/10.1186/s12933-018-0739-3
Gude, F., Díaz-Vidal, P., Rúa-Pérez, C., Alonso-Sampedro, M., Fernández-Merino, C., Rey-García, J., . . . Gonzalez-Quintela, A. (2017). Glycemic variability and its association with demographics and lifestyles in a general adult population. Journal of Diabetes Science and Technology, 11(4), 780–790. https://doi.org/10.1177/1932296816682031
Hammer, M., Storey, S., Hershey, D.S., Brady, V.J., Davis, E., Mandolfo, N., . . . Olausson, J. (2019). Hyperglycemia and cancer: A state-of-the-science review. Oncology Nursing Forum, 46(4), 459–472. https://doi.org/10.1188/19.ONF.459-472
Hammer, M.J., Casper, C., Gooley, T.A., O’Donnell, P.V., Boeckh, M., & Hirsch, I.B. (2009). The contribution of malglycemia to mortality among allogeneic hematopoietic cell transplant recipients. Biology of Blood and Marrow Transplantation, 15(3), 344–351. https://doi.org/10.1016/j.bbmt.2008.12.488
Hammer, M.J., D’Eramo Melkus, G., Knobf, M.T., Casper, C., Fletcher, J., & Cleland, C.M. (2016). Glycemic status and infection risk in nondiabetic autologous hematopoietic cell transplantation recipients. Biological Research for Nursing, 18(3), 344–350. https://doi.org/10.1177/1099800415619227
Hammer, M.J., & Voss, J.G. (2012). Malglycemia and cancer: Introduction to a conceptual model. Oncology Nursing Forum, 39(3), E275–E287. https://doi.org/10.1188/12.ONF.E275-E287
Healy, S.J., Nagaraja, H.N., Alwan, D., & Dungan, K.M. (2017). Prevalence, predictors, and outcomes of steroid-induced hyperglycemia in hospitalized patients with hematologic malignancies. Endocrine, 56(1), 90–97. https://doi.org/10.1007/s12020-016-1220-2
Hill, N.R., Oliver, N.S., Choudhary, P., Levy, J.C., Hindmarsh, P., & Matthews, D.R. (2011). Normal reference range for mean tissue glucose and glycemic variability derived from continuous glucose monitoring for subjects without diabetes in different ethnic groups. Diabetes Technology and Therapeutics, 13(9), 921–928. https://doi.org/10.1089/dia.2010.0247
Hill-Briggs, F., Adler, N.E., Berkowitz, S.A., Chin, M.H., Gary-Webb, T.L., Navas-Acien, A., . . . Haire-Joshu, D. (2021). Social determinants of health and diabetes: A scientific review. Diabetes Care, 44(1), 258–279. https://doi.org/10.2337/dci20-0053
Kim, Y., Rajan, K.B., Sims, S.A., Wroblewski, K.E., & Reutrakul, S. (2014). Impact of glycemic variability and hypoglycemia on adverse hospital outcomes in non-critically ill patients. Diabetes Research and Clinical Practice, 103(3), 437–443. https://doi.org/10.1016/j.diabres.2013.11.026
Kobayashi, D., Noto, H., Takahashi, O., & Shimbo, T. (2020). Glycemic variability and subsequent malignancies among the population without diabetes. Diabetes Research and Clinical Practice, 159, 107987. https://doi.org/10.1016/j.diabres.2019.107987
Kovatchev, B. (2019). Glycemic variability: Risk factors, assessment, and control. Journal of Diabetes Science and Technology, 13(4), 627–635. https://doi.org/10.1177/1932296819826111
Kuhlman, P., Isom, S., Pardee, T.S., Burns, C., Tawfik, B., Lamar, Z.S., . . . Klepin, H.D. (2019). Association between glycemic control, age, and outcomes among intensively treated patients with acute myeloid leukemia. Supportive Care in Cancer, 27(8), 2877–2884. https://doi.org/10.1007/s00520-018-4582-6
Lai, Y.R., Chiu, W.C., Huang, C.C., Tsai, N.W., Wang, H.C., Lin, W.C., . . . Lu, C.H. (2019). HbA1C variability is strongly associated with the severity of peripheral neuropathy in patients with type 2 diabetes. Frontiers in Neuroscience, 13, 90. https://doi.org/10.3389/fnins.2019.00090
Lee, K.C., Chung, K.C., Chen, H.H., Cheng, K.C., Wu, K.L., Song, L.C., & Hu, W.H. (2020). The impact of comorbid diabetes on short-term postoperative outcomes in stage I/II colon cancer patients undergoing open colectomy. BioMed Research International, 2020, 2716395. https://doi.org/10.1155/2020/2716395
Lund, J.L., Gupta, P., Amin, K.B., Meng, K., Urick, B.Y., Reeder-Hayes, K.E., . . . Trogdon, J.G. (2021). Changes in chronic medication adherence in older adults with cancer versus matched cancer-free cohorts. Journal of Geriatric Oncology, 12(1), 72–79. https://doi.org/10.1016/j.jgo.2020.04.012
Mandolfo, N., Berger, A., & Hammer, M. (2020). Glycemic variability in patients with gastrointestinal cancer: An integrative review. European Journal of Oncology Nursing, 48, 101797. https://doi.org/10.1016/j.ejon.2020.101797
Mandolfo, N.R., Berger, A.M., Struwe, L., Hanna, K.M., Goldner, W., Klute, K., . . . Hammer, M. (2022). Glycemic variability within 1 year following surgery for stage II-III colon cancer. Biological Research for Nursing, 24(1), 64–74. https://doi.org/10.1177/10998004211035184
Mills, K.T., Bellows, C.F., Hoffman, A.E., Kelly, T.N., & Gagliardi, G. (2013). Diabetes mellitus and colorectal cancer prognosis: A meta-analysis. Diseases of the Colon and Rectum, 56(11), 1304–1319. https://doi.org/10.1097/DCR.0b013e3182a479f9
Monnier, L., Colette, C., & Owens, D.R. (2018). The application of simple metrics in the assessment of glycaemic variability. Diabetes and Metabolism, 44(4), 313–319. https://doi.org/10.1016/j.diabet.2018.02.008
Peyser, T.A., Balo, A.K., Buckingham, B.A., Hirsch, I.B., & Garcia, A. (2018). Glycemic variability percentage: A novel method for assessing glycemic variability from continuous glucose monitor data. Diabetes Technology and Therapeutics, 20(1), 6–16. https://doi.org/10.1089/dia.2017.0187
Rowley, W.R., Bezold, C., Arikan, Y., Byrne, E., & Krohe, S. (2017). Diabetes 2030: Insights from yesterday, today, and future trends. Population Health Management, 20(1), 6–12.
Saito, Y., Noto, H., Takahashi, O., & Kobayashi, D. (2019). Visit-to-visit hemoglobin A1c variability is associated with later cancer development in patients with diabetes mellitus. Cancer Journal, 25(4), 237–240. https://doi.org/10.1097/PPO.0000000000000387
Simon, J.M., Thomas, F., Czernichow, S., Hanon, O., Lemogne, C., Simon, T., . . . Danchin, N. (2018). Hyperglycaemia is associated with cancer-related but not non-cancer-related deaths: Evidence from the IPC cohort. Diabetologia, 61(5), 1089–1097. https://doi.org/10.1007/s00125-017-4540-8
Singh, M., Upreti, V., Singh, Y., Kannapur, A.S., Nakra, M., & Kotwal, N. (2018). Effect of glycemic variability on mortality in ICU settings: A prospective observational study. Indian Journal of Endocrinology and Metabolism, 22(5), 632–635. https://doi.org/10.4103/ijem.IJEM_11_18
Suh, S., & Kim, J.H. (2015). Glycemic variability: How do we measure it and why is it important? Diabetes and Metabolism Journal, 39(4), 273–282. https://doi.org/10.4093/dmj.2015.39.4.273
Von Ah, D., Brown, C.G., Brown, S.J., Bryant, A.L., Davies, M., Dodd, M., . . . Cooley, M.E. (2019). Research agenda of the Oncology Nursing Society: 2019–2022. Oncology Nursing Forum, 46(6), 654–669. https://doi.org/10.1188/19.ONF.654-669
Wang, J., Yan, R., Wen, J., Kong, X., Li, H., Zhou, P., . . . Ma, J. (2017). Association of lower body mass index with increased glycemic variability in patients with newly diagnosed type 2 diabetes: A cross-sectional study in China. Oncotarget, 8(42), 73133–73143. https://doi.org/10.18632/oncotarget.17111
Yang, Y.F., Li, T.C., Li, C.I., Liu, C.S., Lin, W.Y., Yang, S.Y., . . . Lin, C.C. (2015). Visit-to-visit glucose variability predicts the development of end-stage renal disease in type 2 diabetes: 10-year follow-up of Taiwan diabetes study. Medicine, 94(44), e1804. https://doi.org/10.1097/MD.0000000000001804
Yu, Z.B., Zhu, Y., Li, D., Wu, M.Y., Tang, M.L., Wang, J.B., & Chen, K. (2019). Association between visit-to-visit variability of HbA1c and cognitive decline: A pooled analysis of two prospective population-based cohorts. Diabetologia, 63(1), 85–94. https://doi.org/10.1007/s00125-019-04986-8
Zanders, M.M.J., Haak, H.R., van Herk-Sukel, M P, Herings, R.M.C., van de Poll-Franse, L V, & Johnson, J.A. (2018). Changes in glucose-lowering drug use before and after cancer diagnosis in patients with diabetes. Diabetes and Metabolism, 44(1), 22–29. https://doi.org/10.1016/j.diabet.2017.08.004
Zylla, D., Gilmore, G., Eklund, J., Richter, S., & Carlson, A. (2019). Impact of diabetes and hyperglycemia on health care utilization, infection risk, and survival in patients with cancer receiving glucocorticoids with chemotherapy. Journal of Diabetes and its Complications, 33(4), 335–339. https://doi.org/10.1016/j.jdiacomp.2018.12.012




